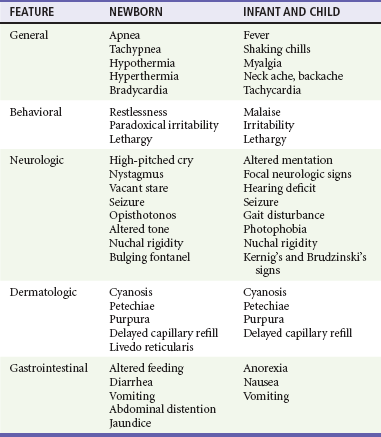
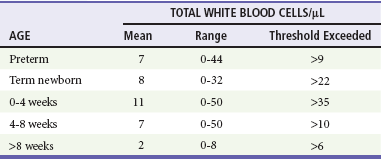
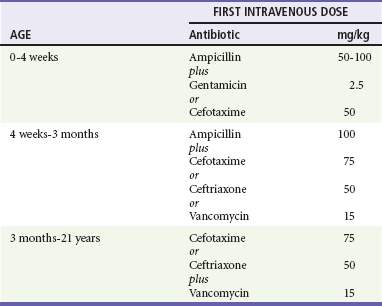
Chapter 175 Despite advances in medical care, acute bacterial meningitis (ABM) remains a potentially life-threatening emergency. Nationwide mortality rates for treated cases are 20 to 30% in neonates and adults and 2% in infants and children.1 With the recommendation in 1991 that all infants, starting at 2 months of age, receive the conjugated vaccine against Haemophilus influenzae type b, the incidence of bacterial meningitis caused by this organism has been reduced in children by more than 99%. However, recent data show that the highest incidence is still found in children younger than 2 months.1,2 Even with the best care, 10 to 30% of all survivors of bacterial meningitis of all types demonstrate persistent, functionally important disabilities.3,4 These include hearing deficits, seizures, learning and behavioral problems, and cognitive impairments. Group B streptococci account for more than three quarters of the cases of neonatal meningitis; Escherichia coli (and other coliforms), Listeria monocytogenes, Streptococcus pneumoniae, Neisseria meningitidis, and H. influenzae are responsible for another one fourth of cases.2 The incidence of early-onset group B streptococcal disease has declined as a result of intrapartum prophylaxis. However, late-onset disease (after 7 days of life) has not. Staphylococcus epidermidis, Staphylococcus aureus, S. pneumoniae, N. meningitidis, group D streptococci, Ureaplasma urealyticum, H. influenzae type b, and nontypable strains are less common pathogens in neonatal infection. L. monocytogenes rarely is the agent of meningitis in neonates but is important because of its prevalence within some immigrant groups, its association with unpasteurized dairy products, and its resistance to cephalosporins.5 Beyond the neonatal period, in the United States, S. pneumoniae, N. meningitidis, and H. influenzae account for 90% of the documented cases of ABM.2 Unusual pathogens include Salmonella species, Campylobacter species, L. monocytogenes, group G streptococci, Francisella tularensis, group B beta-hemolytic streptococci, and several anaerobic organisms.1,2 Recent advances involving the heptavalent pneumococcal conjugate vaccine (PCV7), recommended for all children younger than 2 years, have dramatically reduced the number of cases of bacterial meningitis caused by invasive pneumococcal disease.6 It is anticipated that the newly released 13-valent pneumococcal conjugate vaccine (PCV13) will have an even greater impact on the reduction of invasive pneumococcal disease.7 Bacterial meningitis is thought to result from infection of the respiratory tract, development of bacteremia, invasion of the meninges, and inflammation of the meninges and brain.5 Invasion of the meninges by bacterial pathogens is facilitated by mechanical disturbances. These include recent neurosurgical procedures, such as ventriculoperitoneal shunt placement and lumbar puncture, and skull fracture with a persistent cerebrospinal fluid (CSF) leak. Congenital or developmental central nervous system (CNS) abnormalities also predispose affected patients to bacterial seeding of the CNS. These include intracranial cysts and epidermoid or dermoid tumors, often with an associated congenital dermal sinus track. If bacteria gain entrance into the subarachnoid space, they replicate. Microbial traversal of the blood-brain barrier occurs by microbial interactions with host receptors. Each organism may gain entry in a specific manner. For example, E. coli penetration into the brain involves binding to and invasion of the human brain microvascular endothelial cells, which are part of the blood-brain barrier.8 Host inflammatory mediators and cytokines, including interleukin-1, tumor necrosis factor, and platelet-activating factor, may also be produced and secreted by CNS macrophages and endothelial cells. As a result of an inflammatory response, vascular and parenchymatous cerebral changes occur. These include vasculitis, microthrombus formation, occlusion of venous sinuses, reduced blood flow, increased permeability of the blood-brain barrier, increased intracranial pressure (ICP), diffuse cerebral edema, and intracerebral hemorrhage. Changes involved in microbial invasion of the blood-brain barrier may be different from those involved in the release of inflammatory mediators and cytokines in response to meningitis-causing pathogens.8 In three fourths of children ultimately diagnosed with ABM, the clinical presentation is subacute, evolving during 2 to 5 days. At onset, affected children typically exhibit a variety of symptoms and signs, such as fever, malaise, decreased interest in surroundings, irritability, alteration in sleeping pattern, anorexia, nausea, vomiting, or diarrhea. These findings are nonspecific, seen with equal frequency in children who are suffering from trivial and self-limited illnesses.8,9 If the patient is examined early in the course, physical signs may be subtle or lacking. Children with this insidious presentation have a better prognosis than that of patients with ABM who present with a rapid progression of signs and symptoms. In one fourth of children with ABM, an acute illness with manifestations such as vomiting, fever, and lethargy develops in less than 24 hours. In such patients, the diagnosis is rarely missed.10 Those with fulminant illness exhibit a higher risk for death as well as immediate and long-term complications. The clinical findings with ABM in the neonate are nonspecific and may include altered vital signs, behavioral changes, neurologic aberrations, dermatologic lesions, and gastrointestinal manifestations (Table 175-1). In the first 30 days of life, patients who present to an ED with a temperature of 38° C (100.4° F) or higher are found to have serious bacterial illness (SBI) and ABM in 4 to 15% and 1 to 2% of cases, respectively.11 The absence of fever does not eliminate the possibility of SBI because more than half of neonates with meningitis are afebrile or exhibit hypothermia. Other vital sign changes include tachycardia, bradycardia, tachypnea, and apnea. Apnea with cessation of breathing for longer than 20 seconds may signify either seizure activity or a nonspecific medullary respiratory center dysfunction. Rashes are infrequent manifestations of neonatal meningitis.12 One clue may be generalized pallor accompanied by indistinctly outlined truncal patches of blue discoloration (livedo reticularis). Difficulty in feeding and unusual stooling patterns are nonspecific gastrointestinal features. Nonprojectile vomiting may appear in the course of meningitis, becoming projectile only after ICP has increased. Increased stool volume, irritability during defecation, abdominal distention, hepatomegaly, and jaundice can develop with meningitis. The cardinal features in this age group are fever, headache, vomiting, and stiff neck. Obtundation and lethargy also are common findings with meningitis but are nonspecific. After the first year of life, neck stiffness with difficulty in flexing the neck is reliably seen during the acute phase of meningitis. A patient rarely may exhibit torticollis.13 The clinician can attempt to elicit nuchal rigidity by forced flexion of the neck with the child in the supine position. However, this method is less reliable than attempting neck flexion with the child seated with both legs outstretched. Both of these maneuvers involve passive motion that many children voluntarily resist. More reliable methods that have fewer false-negative and false-positive results involve active neck motion on the part of the child. In a toddler, a distracting object can be used to attract eye contact, and the active range of neck motion can be determined. In addition, the neck stiffness that accompanies ABM may be displayed in both the prone and supine positions when the child’s shoulders are placed at the edge of the examining table and minimal support of the occiput or forehead is provided manually. Presence of Kernig’s sign (flexion of the hip 90 degrees with subsequent pain on extension of the leg) and Brudzinski’s sign (involuntary flexion of the hips and knees after passive flexion of the neck performed with the patient supine) is less reliable. Kernig’s and Brudzinski’s signs are seen in approximately 27% and 51%, respectively, of children 1 to 5 years of age with ABM.14 Indications: Lumbar puncture is indicated in any child in whom bacterial meningitis is suspected (Box 175-1). Patients to be considered candidates for this procedure are those with classic signs and symptoms, such as fever, stiff neck, and photophobia, but in infants and children, classic signs often are absent. In a study by Walsh-Kelly and colleagues, nuchal rigidity was present in only 27% of infants younger than 6 months with bacterial meningitis. By 12 months of age, 71% showed nuchal rigidity; this rate rose to more than 95% by 19 months of age.15 Therefore, the decision to perform a lumbar puncture in children with suspected meningitis should be based primarily on the constellation of presenting signs and symptoms. Nuchal Signs.: A lumbar puncture should be performed in ill children who exhibit nuchal rigidity or Kernig’s sign or Brudzinski’s sign and who have suspected meningitis. These three signs of meningeal irritation can also be seen in non-ABM, such as subarachnoid hemorrhage and trauma. Suspected Sepsis in Young Infants.: A bacterial infection is the most likely etiologic disorder in any infant younger than 3 months who is ill.11 Current practice advocates that the possibility of severe bacterial infection be pursued with an ill young infant. No distinguishing features differentiate sepsis from meningitis in this age group. Because 50% of neonates with acute meningitis are bacteremic at the time of the initial evaluation, and because an intracranial infection subsequently develops in 25% of bacteremic neonates, blood cultures and lumbar puncture should be undertaken concomitantly.16,17 In infants older than 1 month but younger than 3 months, history and physical examination, even if augmented with selective laboratory investigations (excluding lumbar puncture), may fail to identify those infants with SBI. A lumbar puncture should be undertaken unless there is a contraindication as part of the sepsis evaluation in this age group.8,18,19 Toxic Appearance.: A lumbar puncture also should be considered in any child with toxic appearance. Such observations include a blunted response to social overtures, poor perfusion, altered motor tone, and abnormal cry. Febrile pediatric patients of all ages with a toxic appearance have an increased incidence of severe bacterial infections, including ABM.8 When obvious clues to the source of an apparent infectious process exist and an initial lumbar puncture yields normal findings in the face of increasing severity of illness, additional information can be obtained by repeating the lumbar puncture. The CSF composition may change from normal cellularity to marked pleocytosis within as little as 30 minutes.20 Thus later reexamination of the CSF may be invaluable in establishing the nature of an occult intracranial infection. Febrile Illness after Intimate Contact.: All children who have prolonged and close physical involvement with persons found to have a serious bacteremic illness require prompt medical evaluation. In particular, a strong indication for lumbar puncture is the development of fever after intimate contact with high-risk patients with meningococcal disease (higher risk: household contacts, children younger than 2 years, history of direct exposure to secretions from index case or having slept in same dwelling 7 days before onset of illness, and passengers seated next to an index case on a flight longer than 8 hours) or Haemophilus disease (higher risk: household with at least one contact younger than 4 years who is either unimmunized or poorly immunized, a child younger than 12 months who has not received the primary series of immunizations, an immunocompromised child of any age regardless of H. influenzae type b immunization status, and a child who attends daycare where two or more cases have been seen in a 60-day period). Even in the absence of pleocytosis, the emergency physician should consider admission and antibiotic treatment when a child has been exposed to meningococcemia and is symptomatic (e.g., fever, headache) as absence of meningitis is a poor prognosis in this disease. Febrile Seizures.: Children 6 months to 5 years of age who have experienced a simple febrile seizure and who appear well (alert, active, playful) are extremely unlikely to have meningitis. In general, patients who exhibit complex features of the febrile seizure are at increased risk for meningitis. Lumbar puncture should be considered in patients who have characteristics that increase their risk for ABM or who show signs and symptoms of ABM. The historical features are age between 6 and 12 months; exposure to another child with meningitis or serious bacterial infection; deficiency in H. influenzae or S. pneumoniae immunizations, or immunization status cannot be determined; and pretreatment with antibiotics. Children with febrile seizures who exhibit any clinical sign associated with increased risk for intracranial infection, including lethargy, decreased motor tone, doll’s eye sign, inability to fix and follow, decreased response to painful stimuli, nuchal rigidity, full fontanel, petechiae, and poor skin perfusion, should undergo lumbar puncture.21 Fever and Petechiae.: Trivial to life-threatening infectious disease can cause fever and petechiae. No current optimal strategy has emerged for evaluation of febrile children with petechial eruptions. Well-appearing febrile children with localized petechiae, especially above the nipple line and with no purpura, are unlikely to have invasive bacterial disease. Patients at highest risk for invasive bacterial disease are more likely to appear ill or to have generalized petechiae and purpura or signs of meningeal irritation.22 In the high-risk group, unless it is contraindicated by coagulopathy, lumbar puncture should be performed to exclude concurrent meningitis.23 Sepsis Suspected in an Abnormal Host.: Immunocompromised pediatric patients are at risk for the development of both opportunistic infections and invasive illness from pathogens common to all children. Immunologically incompetent children may not exhibit the typical manifestations of intracranial infections, including nuchal rigidity. However, meningitis should be suspected in an immune-impaired child who experiences only a change in mental status. CSF parameters in these patients are less sensitive indicators of the presence of bacterial meningitis compared with normal host response to a bacterial invasion of the meninges. A lumbar puncture nonetheless should be performed in the process of excluding meningitis in a compromised host. Penetration of Dura.: Fracture through the paranasal sinuses or anterior or middle cranial fossa and penetrating nasal injury or instrumentation may lead to dural tears. Such defects constitute a potential portal of entry for microorganisms into the CNS. Evidence of CNS infection generally is seen within 2 weeks of the initial injury, but clinical manifestations may be delayed for years. Persistent CSF otorrhea or rhinorrhea may be encountered but is not a clinical prerequisite for post-traumatic meningitis. When patients with a recent or remote history significant for craniofacial trauma develop any constellation of symptoms suggestive of meningitis, lumbar puncture should be performed after a computed tomography (CT) scan. Acute Hearing Loss.: Hearing loss has been identified in up to one third of patients with ABM. Hearing impairment may be identified in the acute phase or become apparent only in the post-meningitic period. Of significance, hearing loss may precede the onset of systemic complaints and herald the appearance of meningitis. This is especially true for patients with an inner ear fistula or basilar skull fracture. Acute hearing loss should be an indication for lumbar puncture in patients with traumatic injury, which may have directly inoculated organisms into the labyrinth or basilar subarachnoid space, and in patients who have symptoms suggestive of meningitis. Contraindications: Most children with ABM exhibit some mental status changes, but rapid onset and progression to deep coma (Glasgow Coma Scale score of less than 8) usually reflect increased ICP, and lumbar puncture is contraindicated.24 Herniation of the temporal lobes through the tentorium or herniation of the cerebellar tonsils through the foramen magnum rarely occurs in patients with meningitis and increased ICP. Focal neurologic signs suggestive of an abscess are further contraindications to lumbar puncture in children. Positioning and Precautions: When it is not contraindicated, emergency lumbar puncture with CSF examination should be performed to confirm a presumptive diagnosis of ABM. Patients with suspected bacterial meningitis traditionally were placed in the lateral decubitus position with the spine flexed and the knees drawn upward toward the chest, with shoulders and back perpendicular to the table. Studies with ultrasonography have called this positioning into question and reveal that the interspinous space is greatest in the sitting position with the hips flexed, and neck flexion did not result in increase in the interspinous space.25 These data reveal that forward head flexion may not be necessary. Opening pressure should be obtained if possible with the patient in the lateral decubitus position. In circumstances in which opening pressure measurement cannot be obtained, an alternative position is sitting with the thighs flexed toward the abdomen. A standard CSF examination includes bacterial culture and Gram’s stain from tube 1, protein and glucose assessment from tube 2, and blood cell count from tube 3. If there is not an adequate amount of CSF collected, the most important is the specimen sent for culture and Gram’s stain. In cases of culture-proven bacterial meningitis, up to 6% of patients have normal glucose and protein levels, few white blood cells, and negative result on Gram’s staining. Patients who have received antibiotics before their first lumbar puncture merit special mention. Although the CSF can be sterilized after the administration of antibiotics in the ED, especially in cases of pneumococcal and meningococcal disease, the CSF profile often is unaffected for more than 12 to 24 hours after therapy.6 Glucose.: The CSF glucose concentration should be interpreted in relation to a concomitant serum glucose value. The normal steady-state ratio of CSF glucose to serum glucose is approximately 0.6. Hypoglycorrhachia, defined as a ratio below 0.4, is characteristically found in cases of ABM with common pathogens and with Mycobacterium tuberculosis. Hypoglycorrhachia also may be associated with viral meningitis, although CSF glucose concentration in such cases typically is normal. Protein.: The normal CSF protein range is 55 to 80 mg/dL in newborns (65-150 mg/dL in preterm babies) and 5 to 40 mg/dL in children. Normal to modest elevations in protein concentration occur in the course of viral meningitis. Higher levels of protein are encountered with ABM. After a traumatic lumbar puncture, each 800 to 1000 red blood cells elevate the protein level by 1 mg/dL. Cellularity.: A representative cellularity profile is presented in Table 175-2. Unfortunately, these numbers have been largely derived from healthy or nonsystemically ill children.26 The standard “panic values” that have been quoted often are more than 2 SDs above the mean or are figures that are seen in only 5 to 10% of studied populations. Cellularity that exceeds these typical threshold values can be seen with conditions that indirectly influence the CNS; however, such cases should be considered to represent possible ABM and treated accordingly. These conditions include generalized seizure, shigellosis, and parameningeal foci of infections such as otitis media, sinusitis, and mastoiditis. Patients with suspected sepsis or with foci of infection distant from the CNS, such as pneumonia, also may have increased cellularity. Classically in ABM, the total CSF white blood cell count ranges from 1000 to 20,000/mm3. A white blood cell count higher than 2000/mm3 is present in more than one third of cases of ABM. Significant pleocytosis also can accompany aseptic meningitis in as many as 95% of cases.27 Of note, the practice of “correcting” for white blood cells in bloody CSF samples may have little scientific support. Underestimation of the white blood cell count may occur in the setting of culture-positive meningitis. Furthermore, consideration should be given to other illnesses, such as HSV encephalitis, in which an increased number of red blood cells may be seen in the CSF.5 Gram’s Stain.: The probability of visualizing bacteria with Gram’s staining depends on the number of bacterial organisms present at the time of sampling.8 One fourth of the smears yield positive results with 103 or more colony-forming units (CFUs) per milliliter; 60% of the smears show positive results with 103 to 105 CFUs/mL, and 97% show positive results with 106 CFUs/mL. A positive result on Gram’s staining necessitates immediate antibiotic therapy, even in the absence of increased white blood cells and protein levels or decreased glucose level in the CSF. Antigen Detection.: Commercial antigen detection kits are available for the common pathogens causing ABM. Microbial antigens in CSF are detected by coagglutination, latex agglutination, countercurrent immunoelectrophoresis, enzyme-linked immunosorbent assay, and centrifugation-augmented solid-phase immunoassay. Because antigens remain measurable after antibiotic treatment, antigen detection proves to be most effective in cases of partially treated meningitis or in patients in whom the CSF Gram’s stain and culture results are negative and ABM is strongly suspected. In any meningitic state, either untreated or pretreated, the CSF antigen detection kits (commercially available to detect multiple organisms in one packaged product) are most sensitive when more than 500 white blood cells are present in the CSF. The antigens typically are present at high levels in the CSF for several days, even after parenteral antibiotics have been initiated. A positive CSF assay result provides reliable bacteriologic diagnosis. A negative antigen test result cannot exclude the diagnosis of ABM. Cytokines.: Assayed concentrations of C-reactive protein, various interleukins, tumor necrosis factor, and prostaglandins are elevated with ABM. These assays require sophisticated techniques and expensive equipment and take several hours to complete. For these reasons, the assays are of little utility in the ED setting. Any of the manifestations of ABM can be expressed in a single patient. The most common presentation involves a combination of thermal instability, altered behavior, blunted mental status, and neck stiffness. The differential diagnosis for this constellation comprises infectious, metabolic, traumatic, and miscellaneous conditions (Box 175-2). Many conditions cause a child to appear ill. Any infectious agent can cause clinical illness in children, particularly in the first months of life. Infants with infectious disease exhibit altered affect, including significant changes in mental status, strongly suggesting an intracranial pathologic process. Beyond infancy, children may exhibit a toxic appearance with any focal infection or with extensions from a primary focus leading to systemic invasion.10 In addition to infection, a change in mental status may be noted with endocrinopathy, hypoglycemia, electrolyte imbalance, metabolic disease, uremia, seizure, unintentional trauma, abusive head injury, intussusception, or exposure to toxins. In the first few years of life, the signs and symptoms of bacterial and viral meningitis can be indistinguishable.28 Beyond 1 year of age, a majority of children with viral meningitis have a relatively mild illness compared with children with ABM. Fever and retro-orbital or frontal headache are frequent symptoms of both diseases. Gastrointestinal disturbances, such as anorexia, nausea, and diarrhea, are more common with viral meningitis. Generalized myalgia also may be a feature, and patients often complain of neck ache or neck stiffness. However, reduction in active neck flexion is uncommon. Modest elevation of PMNs in CSF specimens coupled with normal glucose level, normal to slightly elevated protein concentration, and negative result on Gram’s staining is anticipated with viral meningitis. These laboratory findings can confirm a clinical suspicion of viral meningitis. However, atypical features, such as hypoglycorrhachia and significant pleocytosis, can be found in 20% of encounters. This blurs the distinction between the two states and may alter management. Decision rules have been proposed to distinguish viral from bacterial meningitis, but their usefulness in clinical practice may be limited if they cannot identify every case of ABM. Nigrovic and colleagues, however, demonstrated a low risk (0.1%) for bacterial meningitis in children who lack the following criteria: positive result of CSF Gram’s stain, CSF absolute neutrophil count of at least 1000 cells/µL, CSF protein concentration of at least 80 mg/dL, peripheral blood absolute neutrophil count of at least 10,000 cells/µL, and history of seizure before or at the time of presentation.27 A major consideration in the choice of antibiotics is the emergence of antimicrobial-resistant organisms, including S. pneumoniae resistant to second- and third-generation cephalosporins and gram-negative bacilli resistant to many β-lactam drugs. Thus empirical treatment in areas where there are resistant strains of S. pneumoniae needs to include vancomycin.8 The four clinical categories of patients and their management are as follows. Nontoxic, low risk: In a non-pretreated patient with no evidence of systemic toxicity in whom the clinical picture is suggestive of viral meningitis, if the CSF indices confirm a clinical suspicion of viral meningitis (negative result on Gram’s stain, few white blood cells in the CSF, and normal CSF protein and glucose levels), antibiotics can be withheld in consultation with the patient’s primary care physician or a neurologist. Another rarely considered option is to discharge the patient and to schedule repeated lumbar puncture within 12 hours after antibiotic therapy. These strategies are not without risk because it often is difficult to distinguish viral meningitis from other infections, such as Rocky Mountain spotted fever and ABM. If biochemical parameters or cytologic assessment findings resemble those in ABM, it is appropriate to begin empirical antibiotic therapy and to hospitalize the patient for observation. Nontoxic, high risk: A patient with no evidence of toxicity but with high-risk historical factors (such as pretreatment with antibiotics, exposure to an invasive organism [H. influenzae or N. meningitidis], or age younger than 1 year) prompting increased concern for ABM requires blood culture and lumbar puncture for CSF analysis. If the spinal fluid is cloudy, antibiotic therapy should begin immediately rather than being withheld pending complete CSF analysis. In symptomatic patients exposed to a person with meningococcemia, appropriate management consists of a full range of cultures, empirical antibiotic therapy, and hospitalization for observation until the results of all cultures are known because the absence of meningitis does not rule out the presence of meningococcal disease. Critical, stable: For the patient with classic signs of ABM who has a protected airway, adequate ventilation, normal perfusion, and no evidence of coagulopathy and is assessed as being in critical but stable condition, a blood culture with phlebotomy and a second culture with placement of an intravenous catheter, urinalysis and culture, blood chemistries, and CBC are recommended, after which a lumbar puncture is performed. Antibiotics should be administered immediately after lumbar puncture and before the results of the CSF or other studies become available from the laboratory. Critical, unstable: A patient with a CNS syndrome, abnormal vital signs for age, unprotected airway, ongoing seizure activity, focal neurologic deficit, or coagulopathy is considered to be in critical and unstable condition. In these patients, the clear risk of an adverse outcome (i.e., respiratory failure) as a result of diagnostic lumbar puncture is magnified, especially if the procedure is carried out before the patient is stabilized. Although the cause and effect relationship is controversial, patients with clinical signs of increased ICP may experience fatal cerebral herniation (even if CT findings are normal) after a lumbar puncture.24 Two blood culture samples and blood and urine specimens for antigen detection should be obtained. Antibiotics should be administered and the lumbar puncture deferred. ED antimicrobial treatment decisions are empirically based. The choice of agents should be based on knowledge of the prevalent organisms responsible for intracranial infections and the regional patterns of their antimicrobial susceptibility. The initial regimen chosen for treatment should be broad enough to provide coverage for the various pathogens typical for the age group being treated (Table 175-3).8 In the newborn, no single antibiotic has bactericidal activity against all of the possible organisms commonly encountered. The most widely used combination therapy consists of ampicillin with an aminoglycoside or ampicillin plus cefotaxime, which is equally effective. For infants older than 1 month and younger than 3 months, in the absence of any evidence for presence of unusual organisms, conventional therapy is ampicillin and a third-generation cephalosporin. Beyond the age of 3 months, monotherapy with a third-generation cephalosporin provides adequate coverage, except in cases involving resistant strains of S. pneumoniae. When gram-positive cocci are identified on the CSF Gram’s stain, the combination of a broad-spectrum cephalosporin (e.g., cefotaxime, ceftriaxone) and vancomycin is now routinely recommended.8 The role of dexamethasone therapy for ABM has long been the focus of clinical interest. Consensus opinion suggests that for infants beyond 8 weeks of age, dexamethasone may mitigate some neurologic sequelae.1 Use of dexamethasone is not without risk. The most commonly reported deleterious effect of dexamethasone is gastrointestinal bleeding. The anti-inflammatory effects of dexamethasone have led to a false sense of clinical improvement, overshadowing the failure of the chosen antibiotic to eradicate an invasive organism. Reduced penetration of vancomycin with dexamethasone therapy has been demonstrated.29 As a potentially universal distribution of cephalosporin-resistant and penicillin-resistant S. pneumoniae approaches, reducing the penetrating effect of vancomycin may cause undue harm. In summary, it is best to adhere to the latest recommendations of the American Academy of Pediatrics (AAP) and to limit the use of dexamethasone to the presumptive treatment of pneumococcal meningitis in infants who are older than 6 weeks after consideration of potential benefits and risks. The AAP recommendation is that dexamethasone may also be beneficial for infants and children with H. influenzae meningitis to decrease the risk of neurologic sequelae, including hearing loss, if it is given before or concurrently with the first dose of an antimicrobial agent.8,30 The issue of empirical treatment of a neonate or young infant (younger than 3 months) with acyclovir is controversial because identification of infected infants is challenging. Acyclovir should be administered in an ill or febrile infant with a history of maternal HSV infection, presence of vesicles on the skin, seizure, or focal neurologic signs. Use of acyclovir also should be considered in atypical presentations of sepsis or meningitis.5 The dose of acyclovir is 20 mg/kg intravenously every 8 hours for 21 days in full-term immunocompetent infants (<35 weeks postconceptual age: 40 mg/kg/day divided q12hr IV for 14-21 days and ≥35 weeks postconceptual age: 60 mg/kg/day divided q8hr IV for 14-21 days). The dose for immunocompromised infants is 750 to 1500 mg/m2/24 hours divided every 8 hours for 7 to 14 days. PCR assay for HSV should be performed in these cases. Treatment may be initiated on the basis of the criteria outlined earlier before results of PCR tests. The young (<5 years), immature nervous system is remarkably susceptible to seizures. Early in development, excitatory activity predominates and inhibitory systems are undeveloped.32 This is known as the period of vulnerability. A paucity of synaptic connections and alterations in the synthesis of neurotransmitters also may play a role. Seizures and Brain Damage: It is well known that children with epilepsy are at a significant risk for cognitive impairment and behavioral abnormalities. It is difficult to distinguish the relative contributions of the effect of the seizures from the underlying CNS disease and from the effect of anticonvulsants. A single prolonged seizure (>30 minutes) has been shown to damage the brain, particularly the temporal lobes and hippocampus.33 In addition, a growing body of evidence points to the lasting effect of repetitive, brief seizures in early childhood.34 Classification of Seizure Type Seizures are classified into two main types: partial (consciousness is maintained) and generalized (consciousness is lost) (Box 175-3). There are two types of partial, or focal, seizures: complex and simple. In complex partial seizures, the patient experiences a change in level of awareness and may exhibit bizarre behaviors, including staring, lip smacking, wandering, or picking at clothing. In simple partial seizures, the patient experiences no change in mentation. An epileptic syndrome is characterized by a triad of age at onset, seizure type, and electroencephalographic findings. Identification of the epileptic syndrome provides information on prognosis and informs management decisions. Several of the more common epileptic syndromes of infancy and childhood are reviewed (Box 175-4). Infantile Spasms: Infantile spasms are manifested during the first year of life with rapid flexor or extensor spasms that appear in clusters. The electroencephalogram (EEG) shows hypsarrhythmia, an abnormal pattern characterized by high-voltage slow waves and disorganized spike activity. Approximately two thirds of children with infantile spasms have an underlying CNS disorder, such as a congenital brain malformation or tuberous sclerosis. The outcome is poor; only half will attain remission of seizures, and the large majority will be mentally retarded. Treatment is notoriously difficult. In the United States, a course of ACTH has traditionally been the treatment of choice; prednisolone treatment is being used with increasing frequency.35 Vigabatrin was recently approved by the U.S. Food and Drug Administration (FDA) for the treatment of infantile spasms and is particularly useful in the setting of tuberous sclerosis. Childhood and Juvenile Absence Epilepsy: Childhood and juvenile absence epilepsy begins between 4 and 12 years of age and is characterized by frequent absence seizures; generalized convulsive seizures occur in up to 50% of the patients. The diagnosis is frequently missed for prolonged periods; the parent or physician may assume the child is daydreaming or purposely not responding. Bursts of spike-and-wave activity at 3/second on the EEG during an absence spell are indicative of this syndrome. Hyperventilation can trigger absence seiziures and can be used to diagnose absence epilepsy. Treatment is with ethosuximide; if convulsions also occur, valproic acid or lamotrigine are used instead.36 The prognosis is excellent, and the majority of children outgrow this disorder by 16 years of age. Benign Epilepsy with Centrotemporal Spikes: This epilepsy syndrome typically begins between 3 and 13 years of age. The seizures occur most commonly during sleep and involve the facial muscles; secondary generalization frequently occurs. The typical electroencephalographic pattern is one of central temporal (rolandic) spikes, particularly during sleep. The prognosis is excellent; the seizures typically cease by 16 years of age. Considerable controversy exists as to whether anticonvulsant treatment of a patient with brief, infrequent seizures is warranted.37 Lennox-Gastaut Syndrome: Developmental disability, multiple seizure types and a “slow” spike and wave pattern on EEG in a young child (2-5 years) are the characteristics of Lennox Gastaut Syndrome. Infantile spasms commonly evolves into Lennnox Gastaut. Valproic acid, lamotrigine, topiramate, felbamate, and rufinamide have shown promise for the treatment of Lennox-Gastaut syndrome,38 but treatment is notoriously difficult. Febrile Seizures: A febrile seizure is defined as a seizure occurring in the presence of fever without CNS infection or other cause. A simple febrile seizure is generalized, lasts less than 15 minutes, and occurs in a neurologically and developmentally normal child between 6 months and 6 years of age.39 Simple febrile seizures typically occur early in the course of the febrile illness. Temperature does not need to be markedly elevated; almost half the children with a febrile seizure have a documented temperature below 39° C. Complex febrile seizures are diagnosed when multiple seizures occur during the same illness, the seizures are prolonged (longer than 15 minutes), or the seizures have a focal component. Febrile seizures are common, occurring in 2 to 5% of children. Thirty percent of children with a simple febrile seizure will have a recurrence; of these, half will have a third event.40 The younger the age at onset, the higher the likelihood of recurrence. Children with simple febrile seizures have a 2 to 3% chance for development of epilepsy (compared with a 1% rate of epilepsy in the general population). Those with complex febrile seizures have a significantly higher risk. Treatment with long-term anticonvulsants does not affect the later risk of epilepsy.41 Rectal diazepam (Diastat) is safe and effective for the termination of prolonged or repetitive febrile seizures and should be available for home use in the child with recurrent, prolonged febrile seizures.42 Long-term anticonvulsant use is rarely warranted.39 The use of antipyretics, such as acetaminophen and ibuprofen, does not decrease the likelihood of recurrence.43 Reassurance and education are the mainstays of management (see the section on management of febrile seizure and the discussion about lumbar puncture). Neonatal Seizures: The frequency of seizures is higher in the first month of life than at any other time in childhood. Neonatal seizures may be subtle; apnea, sustained eye deviation, chewing, or limb bicycling movements may be the only apparent signs. There is a high incidence of subclinical electrographic seizures.44 Focal clonic movements are often associated with an underlying structural lesion. Although there are many causes of neonatal seizures, a relatively few account for a majority of cases. These include hypoxic-ischemic encephalopathy, intracranial infection, congenital brain malformation, cerebrovascular events, and metabolic disturbances, particularly hypoglycemia and hypocalcemia (Box 175-5). Although inborn errors of metabolism are rare, early treatment can be lifesaving.
Neurologic Disorders
Acute Bacterial Meningitis
Neonates
Infants and Children
Principles of Disease
Clinical Features
Neonatal Period (Up to the Age of 1 Month)
One Year to 5 Years
Diagnostic Strategies
Standard Cerebrospinal Fluid Interpretation
Other Cerebrospinal Fluid Tests
Differential Considerations
Viral Meningitis
Management
Antibiotic Therapy
Steroid Therapy
Acyclovir
Seizures
Principles of Disease
Clinical Features
Classification of Epilepsy: Epileptic Syndromes