DEFINITION
This chapter defines mechanical neuraxis injury as fulfilling one of two criteria. The first, direct mechanical injury, encompasses those circumstances in which a needle or catheter directly damages the spinal vasculature, the spinal cord, or a spinal nerve. The second way in which mechanical damage is defined is indirect mechanical injury. In these circumstances, a mass lesion competes for area within the spinal canal, epidural space, or subarachnoid space and exerts sufficient pressure on blood inflow and/or outflow to cause spinal cord ischemia. Examples of such space-occupying lesions include hematoma, abscess, epidural fat, and spinal stenosis from bony overgrowth or soft tissue hyperplasia1 (Box 10-1).
BOX 10-1 Causes of Mechanical Injury to the Spinal Cord
Direct needle or catheter injury
Spinal cord injury
Spinal nerve injury
Spinal vasculature injury
Anterior spinal artery syndrome
Indirect spinal cord injury
Intradural mass lesions
Extradural mass lesions
Epidural hematoma or abscess
Epidural lipomatosis or tumor
Spinal stenosis
Positioning-associated injury
 SCOPE
SCOPE
Neuraxis injury consequent to mechanical trauma is a decidedly rare event, and therefore reliable incidence data do not exist. Awareness of these complications comes from isolated case reports and large surveys of regional anesthesia complications. In the American Society of Anesthesiologists (ASA) Closed Claims Study,2 approximately half of major nerve damage claims were the direct result of neuraxis injury from hematoma, abscess, direct trauma, or vascular insults. Only 12 of 84 (12%) neuraxial injuries were associated with anterior spinal artery syndrome (ASAS) or spinal cord infarct; while 25% of permanent spinal cord injuries during chronic pain management procedures were associated with direct needle trauma.2 A recognized limitation of the Closed Claims Study is that denominators are unknown, and therefore precise incidences cannot be determined. This methodology may also suffer reporting bias consequent to its medicolegal source. French regional anesthesia surveillance data3,4 do not specifically report direct spinal cord or nerve root mechanical injury, but the 95% confidence interval for neurologic injury after 40,640 spinal anesthetics was 3.5 to 8.3 per 10,000 and 0.4 to 3.6 per 10,000 after 30,413 epidural anesthetics.4 ASAS is particularly rare. Only 1 of 57 cases of acute spinal cord ischemia syndrome that presented over a 12-year period to a single referral institution was associated with neuraxial anesthesia.5 In the 2006 to 2007 National Audit Project of the Royal College of Anaesthetists (RCA), spinal cord ischemia occurred in 4 of an estimated 700,000 neuraxial procedures, but it was difficult to determine in these frail, elderly patients whether the etiology was directly attributable to their epidural anesthetic or other factors.6 Although the exact incidence is difficult to determine, it is clear that once neuraxial injury occurs it is often permanent. For instance, 100% of spinal cord infarct claims, 88% of epidural hematoma claims, and 80% of ASAS claims in the ASA Closed Claims database were associated with permanent injury.2 In the 1997 French surveillance study, 15% of all neurologic injuries remained after 3 months4; while the RCA study reported 30% of nerve injuries after neuraxial block remaining after 6 months, and permanence after all ischemic cord injuries.6
In contrast to data on direct spinal cord injury, information regarding spinal cord ischemia as a consequence of mass lesions is more readily available. Analysis of 1990 to 1999 Swedish data for hematoma after neuraxial block reveals an incidence of 1.9 hematomas per 100,000 patients. Furthermore, the incidence varied widely between patient groups—from 1:200,000 for young obstetrical patients to 1:3,600 for elderly females undergoing knee replacement.7 The high rates in the knee replacement group may reflect an increased rate of hematoma consequent to the increased use of powerful perioperative thromboprophylaxis drugs and increased propensity for neuraxial compression because of underlying spinal stenosis, both of which are not typical comorbidities in young parturients. Epidural abscess occurs in 1:1,930 to 5,000 catheters. The higher incidence is associated with immunocompromised patients with longer duration of catheterization and/or anticoagulation.8
 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Direct Mechanical Injury
In case of direct spinal cord injury, the mechanism of injury is seldom known with certainty. Similarly, with the exception of a small number of animal studies, the explanations offered to explain vascular injury (that needles directly injure feeding vasculature or that vascular irritation from needles or injected substances leads to vasospasm) are speculative.9
Spinal Cord Injury
The spinal cord or spinal nerves can be traumatized directly by needle or catheter placement. How this complication can occur and yet at the time be unrecognized by the anesthesiologist or the patient is of particular interest.
That needle- or catheter-induced spinal cord trauma is not reported more frequently is remarkable considering vertebral column surface anatomy and the anatomy of the neuraxis itself. Direct trauma to the spinal cord can occur by several mechanisms. First is misidentification of vertebral interspace level during the performance of spinal anesthesia.7 Traditional teaching states that the caudad terminus of the spinal cord is the L1-2 interspace. However, the pediatric spinal cord typically ends several interspaces caudad to this and the adult spinal cord terminates as low as L4-5 in a small percentage of individuals.10 Moreover, anesthesiologists commonly misidentify the lumbar interspace by plus or minus one interspace.7,11 Particularly in patients in whom surface landmark identification is problematic, the spinal cord may actually reside directly beneath the selected interspace, even when using a low lumbar approach. Two anatomic peculiarities further complicate this situation. First, the ligamentum flavum may incompletely fuse at its most posterior point,12,13 thus failing to provide a sufficient firmness from which to appreciate the loss of resistance and thereby risking unintended meningeal puncture. Second, as one moves cephalad along the vertebral column the distance between the ligamentum flavum and the dura progressively narrows, from 4 to 5 mm in the lumbar area to 1 to 2 mm in the cervical and high thoracic levels.14 Indeed, it is rather surprising that needles or catheters do not contact the spinal cord more frequently than they apparently do. Alternatively, inconsequential spinal cord contact may occur but be unrecognized.
A common misconception is that needle or catheter contact with the spinal cord is always heralded by a painful paresthesia-like sensation.15 Like the brain the spinal cord is devoid of sensory innervation. Case reports document neuroimaging evidence of spinal cord penetration that occurred without recognition by unanesthetized patients undergoing spinal or epidural anesthesia.16–18 In some cases, patients become aware of the presence of a foreign object near the spinal cord when sensory fibers within the meninges19 are stimulated.20,21 Why patients inconsistently recognize20 or fail to recognize16–18 the presence of a needle or catheter touching or penetrating the spinal cord is incompletely understood, although the presence of local anesthetic in the epidural space can significantly lessen the awareness of meningeal puncture.22 Furthermore, medullary penetration does not necessarily result in permanent injury. For example, a patient who sustained unintentional intramedullary spinal catheter placement noted pain upon awakening from general anesthesia, but ultimately suffered no permanent neurologic injury.23 Conversely, an elderly man who suffered permanent injury after placement of a thoracic epidural under general anesthesia was initially unaware of sensory and motor deficits probably because of the infusion of local anesthetic through an unrecognized intramedullary catheter.24
Paresthesia elicited during spinal and epidural needle placement is relatively common (6.3% in a retrospective series of 4,767 spinal anesthetics).25 A needle-induced paresthesia during subarachnoid block has been shown in 87% of occurrences to herald presumably the correct needle placement (as evidenced by the return of cerebrospinal fluid [CSF]).26 Although a paresthesia during block placement has been associated with an increased risk of a persistent paresthesia,25 this relationship is inconsistent.2–4 Moreover, the incidence of paresthesia is vastly greater than the incidence of neuraxial injury. In summary, case reports note that spinal cord penetration with or without injection of local anesthetic may not always be apparent to a patient or anesthesiologist and may not always be associated with neurologic deficit.
Injection of local anesthetic, opioid, or additives into the spinal cord may present a different scenario, in terms of both recognition and injury. Case reports of spinal cord injury associated with injection of anesthetic solution21 suggest that patients are more likely to recognize this event, presumably because volume-induced deformation of the spinal cord results in massive neural discharge as compared to the relatively atraumatic passage of a needle. Permanent neurologic damage may follow intramedullary injection, as a consequence of physical deformity and/or local anesthetic neurotoxicity.24 For example, the intramedullary injection of local anesthetic during intended interscalene brachial plexus block performed on anesthetized patients has resulted in central syrinx formation and permanent paralysis.27 In awake patients, the sequence of painful injection of neuraxial local anesthetic followed by magnetic resonance imaging (MRI) documentation of an intramedullary lesion and permanent neural deficit has been described in several case series and individual case reports.21,28
Spinal Nerve Injury
The spinal nerves represent the joined anterior and posterior nerve roots as they pass through the intervertebral foramen. Temporary and permanent injury to the spinal nerves, presumably from direct needle trauma, has been reported in the French surveillance, the ASA Closed Claims, and other large-scale studies.2–4,6,7 During standard interlaminar approaches, needles and catheters are directed toward the central cord and thus spinal nerve injury is unlikely. However, transforaminal techniques, perivertebral approaches (such as posterior lumbar plexus or paravertebral blocks), or needles that are unintentionally off target could encounter a spinal nerve either within the foramen or as the nerve exits toward the periphery (Fig. 10-1). Stenosis of the intervertebral foramina might further increase the likelihood of nerve root trauma, because a relatively tethered nerve would be less likely to slip away from an approaching needle.
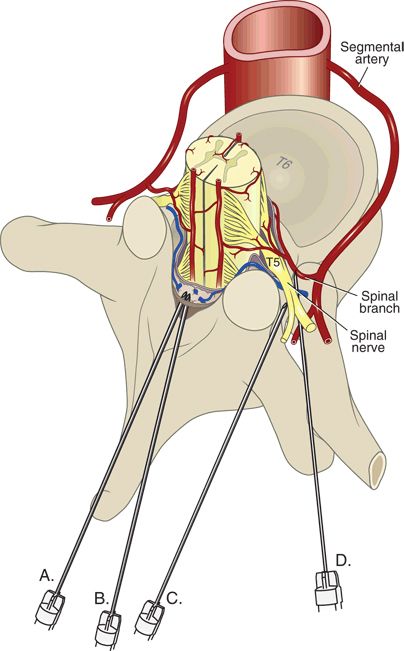
FIGURE 10-1. Potential sites of needle injury to the neuraxis. Midline (A) or paramedian interlaminar approaches (B) are unlikely to be associated with needle contact with a spinal nerve. Intentionally, lateral approaches (C), such as would be used for celiac plexus block or paravertebral block, or transforaminal injections (D) potentially allow needle contact with spinal nerves or spinal arteries.
Vascular Injury
Spinal cord blood supply consists of a variable and incomplete network of arteries and arterioles that branches from larger segmental arteries (Fig. 10-2). The spinal cord is primarily served by three to four major spinal medullary arteries, which are extensions of spinal radicular arteries and supply oxygen and nutrients to three major territories: the cervical cord, the upper and midthoracic cord, and the lower thoracic/lumbosacral cord29 (see Fig. 12-2). The cervical cord arterial supply is typically derived from the vertebral or costocervical arteries, whereas the upper and midthoracic arterial supply comes from a cervicothoracic or a high thoracic segment from the aorta. A single major artery (the radicularis magna or artery of Adamkiewicz) typically supplies the lower thoracic/lumbosacral cord. The cervical and lower thoracic/lumbosacral spinal cord segments are functionally larger than the midthoracic segment, reflecting their innervation of the extremities. Major spinal cord feeder arteries tend to be unilateral (most often left-sided), and, in the case of the radicularis magna, most commonly originate between the T9 and L1 levels30,31 (Chapter 12). The anterior spinal artery and paired posterior spinal arteries are ultimately supplied by this network of segmental reinforcing arteries. Because of their vulnerability as they course laterally along the vertebral bodies and through the intervertebral foramina, it is the segmental arteries and their immediate branches that are most at risk for mechanical injury.
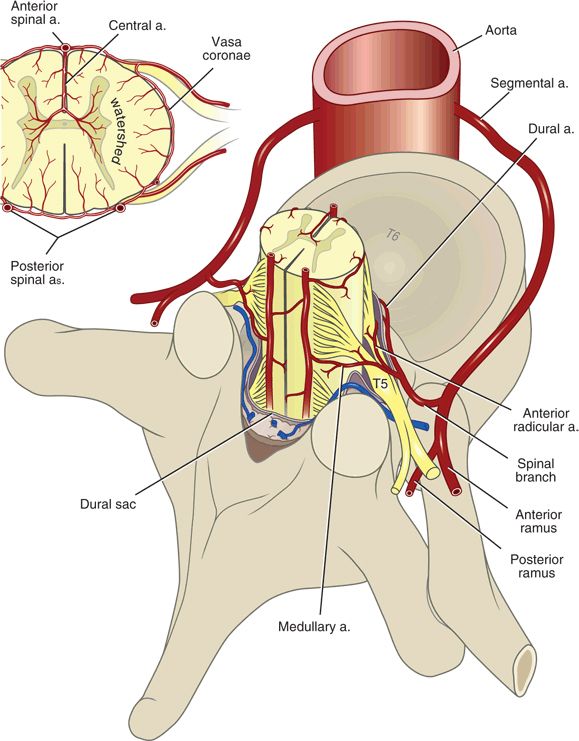
FIGURE 10-2. Arterial vascular supply to the spinal cord. Segmental vessels branch from the vertebral or costocervical arteries, or from the aorta. Most spinal segmental arteries become a single anterior or posterior radicular artery that supplies the spinal nerve roots. Radicular arteries that continue on to the spinal cord are termed medullary arteries, which anastomose across spinal segment levels to become the anterior spinal artery and the paired posterior spinal arteries.
The radicularis magna represents the most commonly implicated artery for direct needle injury. Judging by anatomy, direct injury to this artery should be very rare during spinal or epidural anesthesia because the midline or interlaminar approaches are distant from the intervertebral foramina (through which the major feeder arteries pass). However, anesthetic or pain management techniques that use a more lateral approach to their target (celiac plexus, paravertebral, or transforaminal blocks) may contact a major segmental artery. Needle trauma could damage the artery, or more likely mechanical irritation or injection of substances such as alcohol or phenol could cause vasospasm32 and compromise spinal circulation (Fig. 10-3). Indeed, several case reports of neurologic symptoms after celiac plexus block have postulated chemically induced vasospasm as the mechanism of injury.33,34
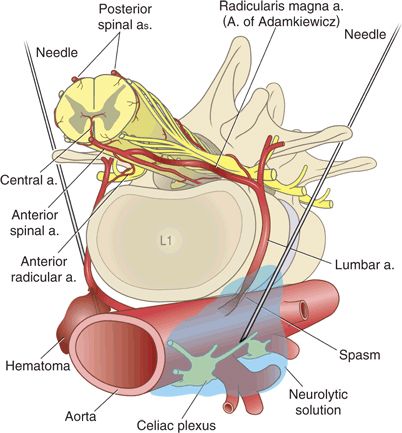
FIGURE 10-3. Needles may directly injure segmental arteries during celiac plexus block or other paravertebral approaches. The left-hand needle illustrates direct vascular trauma with hematoma formation. The right-hand needle precipitated vasospasm as a result of mechanical irritation or chemical irritation from phenol or alcohol. These mechanisms of injury are speculative and have not been proven in humans.



