Neoplasms
Somasundaram Jayabose MD
Neoplasms
INTRODUCTION
This chapter provides a framework for understanding and interpreting the symptoms and signs in cancer diagnosis. Conditions to be explored include extremity and joint pains, lymphadenopathy, abdominal masses, mediastinal masses, headache and neurologic symptoms, spinal cord compression, and hematologic abnormalities.
Other issues surrounding comanagement of the child with cancer are discussed, including chemotherapy, management of neutropenia, and psychosocial issues surrounding diagnosis and treatment. The remainder of the chapter discusses the most common of the childhood cancers, including the leukemias, brain tumors, neuroblastoma, Wilms’ tumor, rhabdomyosarcoma, and the lymphomas.
ANATOMY, PHYSIOLOGY, AND PATHOLOGY
Malignancies in children cause symptoms through various mechanisms. Solid tumors, such as mediastinal tumors and brain tumors, cause symptoms by pressing on adjacent vital structures. Abdominal tumors, such as Wilms’ tumor, can remain silent until detected incidentally by a parent or by a care provider. Neuroblastoma frequently manifests with bone pain from disseminated bone metastases. It may also present with ataxia due to paraneoplastic process. Rarely, some cancers, such as rhabdomyosarcoma, may present acutely with metabolic disturbances, such as hypercalcemia.
EPIDEMIOLOGY
Childhood cancer is a rare disease, with only 1% to 2% of all human cancers occurring in children. Its annual incidence is 130 cases per 1 million children (younger than 15 years). In order of frequency, the most common of the childhood cancers include the leukemias, brain tumors, neuroblastoma, Wilms’ tumor, rhabdomyosarcoma, and the lymphomas (National Cancer Institute [NCI], 2000). The low incidence and the nonspecific nature of the presenting symptoms make early diagnosis of childhood cancer a challenge to the primary care provider. However, including the neoplastic process in the differential diagnosis of persistent, recurrent, or unexplained symptoms may prevent undue delay in diagnosis.
DIAGNOSTIC CRITERIA AND DIAGNOSTIC STUDIES
Extremity and Joint Pains
Malignant diseases, such as leukemia, lymphoma, neuroblastoma, Ewing’s sarcoma, and bone tumors, frequently present as bone or joint pains. Such patients may be mistakenly diagnosed as having rheumatoid arthritis, lupus, Lyme disease, or other rhuematic diseases. Clinical features that are common to both rheumatic and malignant diseases and those that suggest the presence of a malignant disorder are listed in Display 64-1.
DISPLAY 64–1 • Clinical and Laboratory Features of Rheumatologic and Malignant Conditions
Musculoskeletal pain
Fever
Fatigue
Weight loss
Heptomegaly
Arthritis
Features That Suggest a Malignant Process
Nonarticular bone pain
Back pain as a presenting feature
Bone tenderness
Night sweats
Bruising
Pruritus
Abnormal neurologic signs
Anemia, neutropenia, thrombocytopenia
Elevated serum lactate dehydrogenase
Abnormal findings on plain skeletal radiograph, bone scan, or abdominal sonogram
Adapted with permission from Cabral, D. A., & Tucker, L. B. (1999). Malignancies in children who initially present with rheumatic complaints. Journal of Pediatrics, 134(1), 53–57.
Even in the absence of hematologic abnormalities, children with unexplained (by a previous workup) significant (defined as persistent pain that causes a limp or interrupts normal activity) bone and joint pains or musculoskeletal pains must have a bone marrow aspirate study to rule out leukemia or other malignant disorders.
Lymphadenopathy
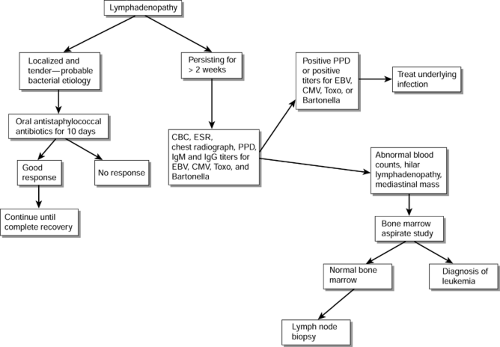
Most cases of bacterial lymphadenitis in children respond to oral antibiotics, and viral lymphadenitis usually subsides in 2 to 3 weeks. Thus, an enlarged lymph node measuring greater than 1 cm and persisting and enlarging for more than 2 to 3 weeks requires further evaluation to determine the etiology. A “shotty” lymph node that diminishes in size between intercurrent illnesses probably does not qualify for workup. Initial screening studies should include the following:
Complete blood count (CBC)
Erythrocyte sedimentation rate (ESR)
Titers for Epstein-Barr virus (EBV), cytomegalovirus (CMV), Bartonella, and Toxoplasma
Intermediate-strength purified protein derivative
Plain chest film
Figure 64-1 outlines a plan for investigation of lymphadenopathy. A markedly elevated ESR is usually suggestive of a systemic illness, probably other than a viral infection.
Presence of neutropenia, thrombocytopenia, mediastinal mass, or hilar lymphadenopathy on plain chest film is very suggestive of malignancy, and such a patient should be further evaluated by a bone marrow aspirate study to rule out leukemia. If the bone marrow aspirate study is negative, a biopsy of the lymph node will be necessary for a definitive diagnosis.
Abdominal and Pelvic Masses
In neonates, more than 85% of abdominal masses are benign. Renal abnormalities, mainly hydronephrosis and cystic dysplasia of kidneys, account for more than 50% of all abdominal masses. In infants and children, fewer than half of all abdominal masses are malignant, and most of the solid renal tumors are Wilms’ tumors. Neuroblastomas account for most of the nonrenal solid tumors in the abdomen. Other abdominal tumors include lymphomas and rhabdomyosarcomas. Common pelvic tumors include rhabdomyosarcomas and ovarian tumors. Most of the cystic lesions are benign.
An ultrasound examination is an ideal screening test, because it can differentiate cystic from solid lesions and requires no contrast or sedation. In addition, ultrasound can detect the presence of a tumor thrombus in the renal vein or inferior vena cava in patients with Wilms’ tumor. Computed tomography (CT) scan is superior to sonogram in demonstrating retroperitoneal structures, including lymph nodes. Optimal CT imaging requires oral and intravenous contrast, however, and young children need sedation to prevent movement artifacts.
• Clinical Pearl
Magnetic resonance imaging (MRI) is superior to CT in visualizing blood vessels, and the additional saggital and coronal views obtained with MRI give better anatomic delineation of the tumor, which may be useful in planning surgery.
Mediastinal Masses
The differential diagnosis of mediastinal masses is outlined in Display 64-2. Children with anterior mediastinal masses often have tracheal compression and present with wheezing or respiratory distress. These symptoms may be exacerbated in the supine position and mistaken for asthma.
DISPLAY 64–2 • Differential Diagnosis of Mediastinal Masses
Anterior Mediastinum
Leukemia
Non-Hodgkin’s lymphoma
Hodgkin’s disease
Teratoma
Thymoma
Lipoma
Thyroid tumors
Middle Mediastinum
Lymphoma
Bronchogenic cyst
Pericardial cyst
Esophageal lesions
Metastatic nodes
Posterior Mediastinum
Neuroblastoma
Ganglioneuroblastoma
Ganglioneuroma
Adapted with permission from Steuber, P. & Nesbit, M. E. (1997). Clinical assessment and differential diagnosis of the child with suspected cancer. In P. A. Pizzo & D. G. Poplack (Eds), Principles and practice of pediatric oncology (3rd ed.) (p. 129). Philadelphia: Lippincott-Raven.
A child with persistent or recurrent wheezing must be evaluated by a plain chest film before undergoing steroid therapy, because steroids can cause significant shrinkage of a mediastinal lymphoma and improvement in the patient’s symptoms, thus falsely reinforcing the diagnosis of asthma.
Anterior mediastinal masses may also compress the superior vena cava, causing some or all manifestations of superior vena cava syndrome. These include orthopnea, headache, dizziness, fainting, plethoric facial swelling, jugular venous distension, papilledema, and pulsus paradoxus.
• Clinical Pearl
Plain chest films are adequate to visualize most mediastinal masses. However, in plain chest films, a mass can be easily obscured by an accompanying pleural effusion, giving a false impression of pneumonia with effusion. A CT scan gives better anatomic delineation between the mass, fluid, and the lung parenchyma.
It is sound clinical practice to obtain a CT scan of the chest in cases of large pleural effusion that may be masking a mediastinal mass. Confirmation of diagnosis requires examination of pleural fluid, bone marrow aspirate study, or biopsy of an enlarged cervical lymph node or the mediastinal mass itself.
Sedation or general anesthesia of a child with anterior mediastinal mass can cause worsening of tracheal compression, resulting in exacerbation of the respiratory distress and even respiratory arrest. Cases of sudden death in the operating room have been reported because of collapse of the airway and difficulty in intubation. Sedation and general anesthesia should be avoided whenever possible and used only by a team of experienced pediatric anesthesiologists in a tertiary care center.
Headache and Neurologic Symptoms
Brain tumors are the most common solid tumors in children. The possibility of a brain tumor should be considered in any child with unexplained neurologic symptoms or signs. The presenting symptoms and signs of brain tumors may be localizing in nature, such as hemiparesis of hemispheric lesions. Nonlocalizing symptoms are secondary to increased intracranial pressure and include irritability, lethargy, headache, vomiting, and behavioral changes. A more complete list of signs and symptoms of childhood brain tumors is given in Display 64-3.
DISPLAY 64–3 • Signs and Symptoms of Brain Tumors in Children
Increased Intracranial Pressure
Morning headache
Vomiting
Visual disturbances
Developmental delay
Regression of intellectual and motor skills
Lethargy, irritability, anorexia
Excessive tiredness
Changes in personality
Poor school performance
Infratentorial Tumors (Brain Stem, Cerebellum)
Disturbance of balance or brain stem function
Ataxia predominantly truncal
Long tract signs
Cranial nerve palsy
Supratentorial Tumors
Subtle loss of consciousness
Transient focal events
Grand mal seizures
Hemiparesis or hemisensory loss
Hypothalamic Tumors
Loss of growth velocity
Diabetes insipidus
Delayed puberty or sexual precocity
Visual loss
Adapted with permission from Tait, D. M., Bailey, C. C., & Cameron, M. M. (1992). Tumors of the central nervous system. In P. A. Voute, A. Barret, & J. Lemerle (Eds)., Cancer in children: Clinical management (3rd ed.). (pp. 184–206). New York: Springer-Verlag.
Note: None of the more recent pediatric oncology resources have content as comprehensive as that provided in this reference. Symptoms and signs of the disease are not likely to change along with advances in science. The reader should not be concerned about the currency of the reference.
Spinal Cord Compression
Children who complain of back pain or weakness of lower extremities should be promptly evaluated for spinal cord compression. MRI is the preferred imaging study because it can demonstrate an epidural mass and any intraparenchymal
spinal cord lesion or compression of the cauda equina. Although rare, neuroblastomas and lymphomas can present with spinal cord compression.
spinal cord lesion or compression of the cauda equina. Although rare, neuroblastomas and lymphomas can present with spinal cord compression.
The provider should treat any suspected spinal cord compression as an emergency, because progression of the lesion can result in irreversible loss of neurologic function. Treatment depends on the underlying diagnosis but usually includes high-dose dexamethasone, laminectomy, radiation therapy, or chemotherapy. Prompt treatment often results in full recovery of neurologic function.
Hematologic Abnormalities
In an otherwise well-looking child, single cytopenia (anemia, thrombocytopenia, or neutropenia) is unlikely to be caused by leukemia.
If the child is ill with systemic symptoms, leukemia should be considered in the differential diagnosis. Children with neuroblastoma, lymphoma, or Hodgkin’s disease may have anemia at presentation. When there is a combination of two or more cytopenias, leukemia must be ruled out by a bone marrow aspirate study. Neutrophilic leukocytosis and microcytic anemia are often seen in Hodgkin’s disease.
When bacterial infections cause very high white blood cell (WBC) counts (leukemoid reaction), there is a high percentage of neutrophils and bands. In leukemia, however, a high WBC count is always associated with a very low percentage of neutrophils, because the increase in the WBC count is due to the presence of leukemic cells (blasts) in the peripheral blood.
• Clinical Pearl
The higher the WBC count, the more easily one can identify leukemic cells in the peripheral blood.
Referral to the Pediatric Oncologist
A provisional diagnosis of cancer can often be made, or at least strongly suspected, by the primary care provider from the results of the preliminary tests, including certain imaging studies. The definitive diagnosis of cancer, however, frequently requires confirmatory studies, such as bone marrow aspirate study in the case of leukemia or histopathologic examination of the tumor tissue in solid tumors. In addition to routine morphologic examination of the specimen by light microscopy, special studies, such as immunostains, cytogenetics, flow cytometric analysis of tumor-specific antigens, cell ploidy, and molecular biologic studies, are often essential for the definitive diagnosis and assessment of prognosis. These confirmatory tests should be done at the referral center under the direction of a pediatric oncologist.
MANAGEMENT
During the course of their treatment, children with cancer are followed closely by the pediatric oncologist. However, the primary care provider continues to play an important role in the management of the child with cancer. This may include comanaging chemotherapy and other drug administration. The provider will almost certainly manage minor intercurrent illnesses, provide well child care, and offer psychosocial support to the child and the family. In addition, the primary care clinician will follow the patient for several years after the treatment is completed. The provider–patient relationship is important for several reasons, not the least of which is knowing the child and his or her history of illness and response to therapy, which can facilitate an early diagnosis of any late effects of cancer therapy, as described in the following section.
Chemotherapy
The primary care provider can play a significant role in the treatment of the child with cancer. This requires developing a close working relationship with the pediatric oncologist. In some settings, the provider can coparticipate in the patient’s cancer therapy, administering selected chemotherapy that has been prescribed by the oncologist. Examples of such agents include vincristine, cytarabine, and dactinomycin. These agents are administered by slow intravenous push through a central venous catheter. In addition, the practitioner may also give intramuscular injections of L-asparaginase and subcutaneous injections of cytosine arabinoside prescribed by the oncologist. These two drugs are commonly used in the treatment of acute lymphoblastic leukemia (ALL). Such participation by the primary provider vastly improves the quality of life for patients and parents by enabling them to receive treatment in a smaller, more familiar setting and by decreasing their visits to an often more distant cancer center.
Side Effects of Chemotherapy
The side effects of chemotherapy can be classified into general and specific. General side effects are those that are common to most chemotherapeutic agents. These include nausea, vomiting, mucositis, hair loss, and myelosuppression. These side effects are seen within hours or days from the administration of chemotherapy and are almost always transient. However, the severity of these side effects is variable. For example, vincristine, L-asparaginase, and bleomycin are much less myelosuppressive than most other drugs. Cisplatin, dacarbazine, and mechlorethamine (nitrogen mustard) are among the most emetogenic drugs, while etoposide and bleomycin are only mildly emetogenic. Vincristine seldom causes any nausea or vomiting.
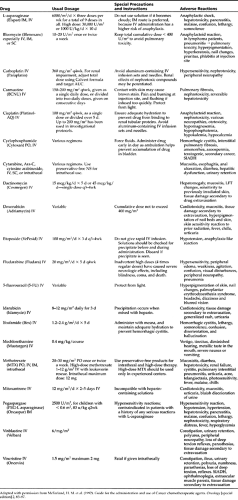
Specific side effects are those that are specific to a particular drug or class of drugs, and they usually involve vital organ systems. Common examples include cardiotoxicity of anthracyclines, nephrotoxicity of platinum compounds, pulmonary toxicity of bleomycin and carmustine (BCNU), and bladder toxicity (hemorrhagic cystitis) of cyclophosphamide and ifosfamide. The reader is referred to Chapter 15, Chapter 27, Chapter 28, and Chapter 49 for more insight into managing care coordination for the child with cancer. Table 64-1 lists the common side effects of the frequently used chemotherapeutic drugs.
Infections
A host of factors, including neutropenia, low immunoglobulin levels, impaired mucosal integrity, and indwelling catheters, compromise the immunity of children with cancer. Lowered immunity makes such children susceptible to serious infections. Gram-negative organisms such as Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae are a particular threat to the neutropenic host. Staphylococcal infections, Pneumocystis carinii pneumonia, and cryptococcal meningitis occur in non-neutropenic hosts as well. Coagulase-negative staphylococci are common pathogens in patients with indwelling central lines. Life-threatening
complications include gram-negative sepsis, pneumonias, and disseminated fungal infections.
complications include gram-negative sepsis, pneumonias, and disseminated fungal infections.
The Neutropenic Patient With Infection
The risk of serious bacterial and fungal infections is significant when the absolute neutrophil count (ANC) is less than 500/mm3, and it is highest when the ANC drops below 100/mm3. The febrile child with an ANC of less than 500/mm3 should be hospitalized for intravenous broad-spectrum antibiotic coverage and close monitoring for complications, such as septic shock. Display 64-4 presents the clinical features that indicate high risk for life-threatening complications in the neutropenic patient.
DISPLAY 64–4 • Features That Indicate a High Risk of Sepsis in the Neutropenic Child With Cancer
Relapsed patients on high-dose chemotherapy
Absolute neutrophil count < 100/mm3
Neutropenia lasting for > 7 days
Temperature of ≥ 103°F
Hypotension
Documented focus of infection (eg, pneumonia, cellulitis)
Mucositis of the gastrointestinal tract, diarrhea
Renal, hepatic, or cardiac dysfunction
Adapted from Freifeld, A. G., Walsch, J. W., & Pizzo, P. A. (1997). Infectious complications in the pediatric cancer patient. In P. A. Pizzo & D. G. Poplack. Principles and practice of pediatric oncology (3rd ed.). (p. 1069–1114). Philadelphia: Lippincott Raven Publishers.
The patient with these risk factors should be managed at a center experienced in the care of immunocompromised patients. Any patient who lacks these high-risk features may be managed by the primary care provider in consultation with the pediatric oncologist. All patients, however, should be hospitalized and treated with intravenous antibiotics until they are afebrile and the culture results are available to guide further management. Even if the cultures are negative and the patient is afebrile, it is preferable to continue the intravenous antibiotics until the ANC is 500/mm3 or greater.
Ceftazidime is commonly used as the initial empiric therapy (pending culture results), because it provides adequate coverage against most gram-negative organisms. However, it is not very effective in anerobic and staphylococcal infections, and changes in antibiotic therapy need to be made if there are clinical clues indicating such infections.
To prevent emergence of vancomycin-resistant Enterococcus, the use of vancomycin should be restricted to proven infections with coagulase-negative Staphylococcus that are resistant to other antibiotics.
In culture-proven Pseudomonas infections, an aminoglycoside, such as gentamycin or tobramycin, should be added to ceftazidime to prevent emergence of resistant organisms. If necessary, imipenem should be substituted for ceftazidime to provide added coverage against anerobic organisms in cases of perirectal abscess, gingivitis, or typhlitis. It may also be preferred in patients who might have resistant organisms because of multiple exposure to previous antibiotic therapy. Refer to the section on fever in the neutropenic child for management information.
The Febrile Non-neutropenic Patient
The non-neutropenic child with a temperature of 102°F or greater warrants careful evaluation, although risk of life-threatening infections is much less than that of the neutropenic child. Physical examination should include a careful search for any evidence of pneumonia or meningitis. Plain chest films and blood cultures from the central line should be done in any patient who does not have a definite focus of infection on physical examination. Indications for hospitalization include ill-looking child, a temperature of 103°F or greater, and radiologic evidence of pneumonia. Outpatient management may be appropriate if the febrile child does not look ill, the temperature is less than 103°F, and there is no evidence of pneumonia. Empiric antibiotic therapy with parenteral ceftriaxone may be used for such a patient pending culture results. Children with implantable subcutaneous catheters (lifeport) are less likely to have central line-related bacteremia compared with those with external catheters.
Respiratory Tract Infections: Otitis Media, Sinusitis, and Pneumonia
In addition to the common bacteria, any gram-positive or gram-negative organism can cause otitis media or sinusitis in immunocompromised children. Oral broad-spectrum antibiotic therapy is sufficient for the non-neutropenic patient. In neutropenic patients, otitis media or sinusitis can be caused by any gram-negative or gram-positive organism.
Febrile neutropenic patients with otitis media or sinusitis should be treated with intravenous antibiotics until results of blood cultures are available, because clinical evidence of otitis or sinusitis does not rule out the possibility of sepsis.
A CT scan or MRI is more sensitive than plain radiograph in diagnosing sinusitis in immunocompromised children. Sinusitis in patients with severe and prolonged neutropenia is often caused by fungal organisms. If a neutropenic patient with sinusitis does not improve within 72 hours of treatment, aspiration or biopsy of the sinus should be performed to identify the causative organism so that specific therapy can be started.
Pneumonia is one of the common infections in immunocompromised children, and diffuse bilateral pneumonia is one of the main causes of death in children with cancer. The causative organisms may vary depending on the type of pulmonary infiltrate (localized versus diffuse) and the presence or absence of neutropenia.
A localized pulmonary infiltrate in a non-neutropenic patient is usually caused by the same bacteria, virus (respiratory syncytial virus [RSV], adenovirus, influenza), or Mycoplasma that cause pneumonia in normal children. Such a patient may be managed by the primary care provider with oral antibiotic therapy if the patient does not look ill and the temperature is less than 103°F. However, less common bacterial agents, such as Mycobacterium tuberculosis, atypical mycobacteria, Legionella, and fungi (Cryptococcus, Histoplasma, and Coccidioides) should also be considered in determining the causative organism.
When the pulmonary infiltrate is diffuse or if the patient is neutropenic, the patient’s condition may worsen rapidly, and the risk of mortality is significant. Such a patient should be treated in the intensive care unit at a tertiary care center.
Whether the infiltrates in the neutropenic patient are localized or diffuse, all organisms should be considered in the etiology:
Gram-positive and gram-negative bacteria, including M. tuberculosis, atypical mycobacteria, and Legionella
Viruses, including RSV, CMV, and influenza
Fungi, including Aspergillus and Candida
Protozoal (eg, P. carinii pneumonia)
• Clinical Pearl
Diffuse infiltrates in a non-neutropenic child are more likely to be caused by viruses (RSV, CMV) and Pneumocystis than bacterial or fungal organisms.
Infections of the Oral Cavity
Common lesions in the mouth of children receiving chemotherapy include thrush, herpes stomatitis, and gingivitis and periodontitis. Thrush is characterized by whitish plaques with slightly raised and indurated borders caused by unchecked colonization by Candida albicans. Mycostatin oral suspension or clotrimazole troches are effective in most patients. Patients who do not respond to these treatments should be treated with oral fluconazole.
Herpes simplex virus (HSV) lesions in the mouth are often multiple and are most commonly seen on the lips and palate. The characteristic finding is a slowly enlarging ulcer (usually measuring greater than 1 cm), with a raised white border and sometimes with small vesicles surrounding the ulcer. The lesions are quite painful, and they affect the patient’s oral intake. Empiric treatment with acyclovir should be started as early as possible to prevent more severe stomatitis. Oral acyclovir (20 mg/kg per dose, four times daily) is adequate for mild lesions. Severe herpes stomatitis requires treatment with intravenous acyclovir. HSV lesions are often
difficult to differentiate from mucositis induced by chemotherapy or by bacterial or fungal pathogens. Thus, empiric antibiotic therapy in a patient with stomatitis should also cover anerobic organisms.
difficult to differentiate from mucositis induced by chemotherapy or by bacterial or fungal pathogens. Thus, empiric antibiotic therapy in a patient with stomatitis should also cover anerobic organisms.
Esophagitis
Retrosternal pain of a burning nature is indicative of esophagitis. In a non-neutropenic child, this may represent a reflux esophagitis. Children with severe chemotherapy-induced vomiting and those who are ill and bed-ridden for a prolonged period are at high risk for reflux esophagitis.
Failure to recognize esophagitis promptly may later result in marked esophageal stenosis, requiring treatment with dilatations.
Esophagoscopy may not be safe in neutropenic patients, because any trauma to the mucosa may aggravate the infection.
Esophagitis in a neutropenic child is commonly caused by C. albicans or HSV. Presence of oral thrush suggests Candida as the etiology for the esophagitis. Absence of thrush, however, does not rule out Candida as the cause of esophagitis. Empiric therapy can be started with oral or intravenous fluconazole and acyclovir. If there is no significant improvement in 2 or 3 days, intravenous amphotericin should be started.
Prevention of Infections in Children With Cancer
Most of the infections in children with cancer are caused by endogenous organisms. Attempts to sterilize the gastrointestinal tract with broad-spectrum antibiotics are not effective in reducing the incidence of infections. In addition, their use may increase the emergence of resistant organisms. Simple hand washing by all hospital personnel and visitors is the most effective prevention against hospital-acquired infections. Strict reverse isolation does not usually add much to hand washing in the prevention of infections. Patients should avoid eating any uncooked food, including fresh vegetables and fresh fruits. High-efficiency particulate air filters may be effective in preventing Aspergillus infections (Freifeld, Walsch, & Pizzo, 1997). Chapter 54 provides a discussion about these filters.
Antimicrobials
Trimethoprim-sulfamethoxazole (TMP-SMZ), administered on 3 consecutive days a week, is very effective in preventing P. carinii infections and is now routinely prescribed for most children on chemotherapy (El-Sadr, Luskin-Hawk, Yurik, et al., 1999). Children who are allergic to TMP-SMZ may receive biweekly intravenous pentamidine or aerosolized pentamidine.
The prophylactic use of fluconazole has been shown to reduce the incidence of disseminated and mucosal candidiasis (Goodman, 1992). However, it is wise to limit the use of fluconazole to periods of severe and prolonged neutropenia, because its excessive use may encourage the emergence of resistant Candida species. Oral acyclovir is effective in preventing reactivation of herpes simplex infections in seropositive bone marrow transplant and in children undergoing intensive chemotherapy for leukemia.
Immunizations: Passive and Active
Irrespective of their history of natural varicella or varicella immunization, children diagnosed with cancer should have their varicella titers tested. Those who do not have adequate titers should receive varicella zoster immune globulin (VZIG) within 96 hours of exposure to chickenpox. Passive immunization with VZIG reduces the incidence of varicella pneumonitis and encephalitis and decreases the mortality rate from between 5% and 7% to 0.5% in patients who are immunocompromised and who have primary varicella infection (Freifeld et al., 1997).
Live virus vaccines, such as measles, mumps, and rubella vaccine and oral polio vaccine, are contraindicated in children undergoing chemotherapy, although measles vaccine has been safely used in children with human immunodeficiency virus.
Siblings of children on chemotherapy should avoid oral polio vaccine because the vaccine virus is transmissible. Live attenuated varicella virus is still not approved for children who are immunocompromised, although studies have shown that it is safe and 85% protective. However, siblings of children on chemotherapy may receive varicella vaccine.
• Clinical Pearl
Patients and siblings of children on chemotherapy may receive inactivated (injectable) polio vaccine.
Although killed vaccines, such as hepatitis B vaccine, and antigens, such as diphtheria-tetanus-pertussis, may be safely administered to immunocompromised children, their immunogenicity may not be optimum. Refer to Chapter 13 for a detailed discussion.
Managing Hematologic Complications of Therapy: Anemia and Thrombocytopenia
Children on cancer chemotherapy are frequently anemic and thrombocytopenic from either chemotherapy or their underlying disease. In most chemotherapy regimens, the nadirs for platelet counts and WBC counts occur 7 to 10 days from the first day of chemotherapy. The counts begin to rise about 10 to 14 days from the completion of therapy. Decrease in the hemoglobin levels is more gradual, because the lifespan of normal red cells is 90 to 120 days.
Red cell transfusions are usually given when the hemoglobin is less than 8 g/dL (hematocrit less than 24%), unless the child is recovering from the chemotherapy and the hemoglobin is expected to rise. When the hemoglobin level falls below 7 g/dL, patients usually have symptoms of anemia, including tiredness, headaches, anorexia, and lack of energy. Transfusion of red cells thus is indicated. One unit of packed red cells (PRBC) is approximately 300 mL. A usual volume used is 15 mL of PRBC per kg of body weight, which will raise the hematocrit by 12% (eg, from 20% to 32%).
• Clinical Pearl
One mL of PRBC per kg body weight will raise the hematocrit by 0.8%.
Platelet transfusions are indicated when the platelet count is less than 20,000/mm3. If there is clinical bleeding (eg, epi-staxis or gastrointestinal bleed), any platelet count less than 50,000/mm3 warrants transfusion. One unit of random platelets (50 mL) per 5 kg of body weight will raise the platelet count by 50,000/mm3. One unit of single donor
platelets (approximately 300 mL) is equal to 6 U of random platelets. Single donor units are preferred for those who receive multiple transfusions.
platelets (approximately 300 mL) is equal to 6 U of random platelets. Single donor units are preferred for those who receive multiple transfusions.
Only irradiated blood products should be used in immunocompromised patients. Only CMV-negative blood products should be used for CMV-negative patients undergoing allogeneic stem cell transplantation from a CMV-negative donor.
Use of Growth Factors
Granulocyte-colony stimulating factor (G-CSF, Filgrastim, Neupogen) stimulates the proliferation of the granulocyte precursors, and accelerates their maturation to neutrophils. A course of G-CSF given soon after a cycle of intensive chemotherapy reduces the duration of severe neutropenia, thus reducing the risk of infection and the duration of hospitalization in patients with infection. It is given in doses of 5 mg/kg daily, starting the day after the completion of chemotherapy, and continued until the ANC reaches 5000/mm3 (which usually takes 10–14 days). It is given as a subcutaneous injection or as an intravenous push through the central line. Blood counts are done twice a week during G-CSF therapy so that treatment can be discontinued once the WBC counts reach high normal values (> 10,000).
Continued administration of G-CSF results in extreme leukocytosis (> 50,000) and may cause bone pains. G-CSF support is indicated only for patients receiving intensive chemotherapy for solid tumors, acute myeloblastic leukemia, or relapsed ALL. G-CSF is usually not used in newly diagnosed ALL because the chemotherapy is not intensive enough to justify its use. However, most patients with relapsed ALL receive intensive therapy, and G-CSF is used to augment the recovery of neutrophils. There is no risk of G-CSF stimulating the proliferation of leukemia cells.
Erythropoietin is a glycoprotein that stimulates the division and differentiation of erythroid progenitors in the bone marrow. It is given in doses of 150 IU/kg three times per week after myelosuppressive chemotherapy. A modest decrease in the transfusion requirement has been noted in patients receiving erythropoietin (Henry, 1995; Wurnig et al., 1996). However, the cost of erythropoietin exceeds the cost of red cell transfusions (Ortega, Dranitsaris, & Puodziunas, 1998).
Follow-up of the Child Cured of Cancer
Approximately 70% to 80% of all children with cancer are cured by modern therapy. However, a significant percentage of these children have long-term side effects of treatment. Growth and development and reproductive function can be impaired. Cardiac or pulmonary dysfunction and secondary malignancies are also among the common late effects of treatment.
Growth and Development
Prepubertal children receiving 3300 cGy (rads) or more of radiation to the spine for the treatment of brain tumors or Hodgkin’s disease have pronounced impairment of linear growth, especially the sitting height. On the other hand, children who get lower doses of radiation (< 3300 cGy), as, for example, for the control of central nervous system (CNS) leukemia, have minimal height loss. Cranial radiation in ALL has minimal risk of growth hormone deficiency (GHD), because the total dose (1800 cGy) and the daily fraction (150 cGy) are low. On the other hand, children receiving radiation for brain tumors (usually < 3000 cGy) have a high risk of GHD. Cranial irradiation for ALL or brain tumors can also cause precocious puberty and decreased adult height. Primary or secondary amenorrhea is seen in one third of patients receiving more than 3000 cGy of radiation for brain tumors (Arlt et al., 1997). Refer to Chapter 60 for information about the management of GHD.
Gonads and Reproductive Function
Alkylating agents (nitrogen mustard, cyclophosphamide, ifosfamide, melphalan, thiotepa, busulfan) cause gonadal damage, particularly in males. Males receiving MOPP regimen (nitrogen mustard, vincristine, procarbazine, prednisone) for Hodgkin’s disease have a very high risk of sterility: greater than 90% with six cycles and 50% with three cycles. Overall, the risk of sterility in males treated with alkylating agents for various cancers has varied from 20% to 66% in various studies. On the other hand, the risk of sterility in females from alkylating agents is much lower.
Nervous System
Cranial irradiation and intrathecal chemotherapy can cause a spectrum of neurologic complications ranging from mild learning disabilities to frank leukoencephalopathy. With modern treatment protocols for ALL, most children receive intrathecal methotrexate alone for CNS prophylaxis, and these patients have only a small risk of neurocognitive defects.
Children who receive cranial irradiation or high doses of systemic methotrexate along with intrathecal methotrexate are at higher risk for neurocognitive sequelae, including learning defects. Infants and young children are more at risk for this complication than older children.
One third of children younger than 5 years receiving cranial irradiation for ALL develop learning difficulties. Children who experience CNS relapse (meningeal leukemia) often receive all three modalities of CNS treatment—systemic methotrexate, intrathecal or intraventricular chemotherapy, and cranial irradiation. Such patients may develop more severe neurologic sequelae, such as leukoencephalopathy, seizures, major motor disabilities, and cranial nerve palsies. Children with malignant brain tumors treated with doses equal to or greater than 54 rads (cGy) of radiation are also at risk for any of these complications.
Cardiac Function
The incidence of clinically evident cardiotoxicity in children has been estimated to be 1.6% from a study of 6493 patients (Krischer et al., 1997). The risk factors for cardiac toxicity include cumulative anthracyclines dose of 550 mg/m2 or greater, single dose of 50 mg/m2 or greater, female sex, age younger than 4 years, African American race, length of follow-up, trisomy 21, and exposure to amsacrine (Krischer et al., 1997; Lipshultz et al., 1991).

Full access? Get Clinical Tree