Figure 14.1
Barium Swallow Study in a patient with achalasia showing a mildly dilated esophagus and the classic “bird’s beak” deformity at the gastro-esophageal junction
3.
Esophageal manometry: Esophageal manometry is the best method to characterize the motility of the upper esophageal sphincter, esophageal body and the LES, and is currently considered the gold standard method for the diagnosis of achalasia. Nowadays, esophageal manometry catheters have been equipped with high resolution capacity and impedance sensors to further correlate esophageal motor function with the bolus clearance capacity. The classic motility pattern includes two requirements: the loss of progressive peristalsis in the body of the esophagus, and failure of complete LES relaxation in response to deglutition [20]. As peristalsis is absent, the contractions recorded at different esophageal levels are simultaneous and usually of low amplitude (Fig. 14.2). Other features that can be seen include elevation of intraluminal esophageal pressure and hypertension of the LES (50 % of the patients) [21]. A subgroup of patients with otherwise typical features consistent with classic achalasia show higher amplitude, active simultaneous contractions of their esophageal body; this manometric pattern has been termed vigorous achalasia [22] and may be manifested as frequently atypical chest pain episodes as by progressive dysphagia.
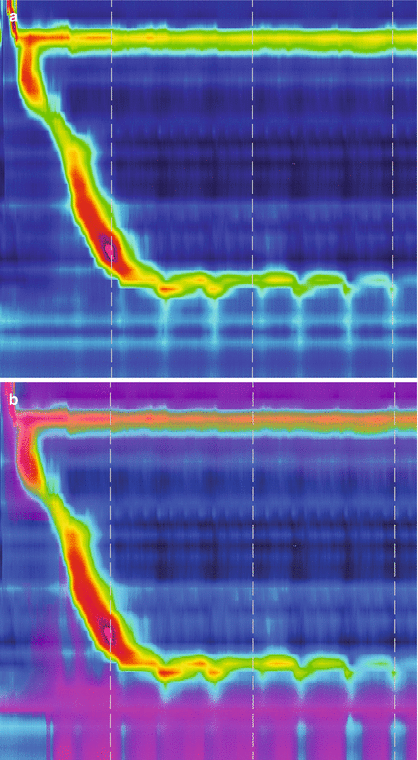
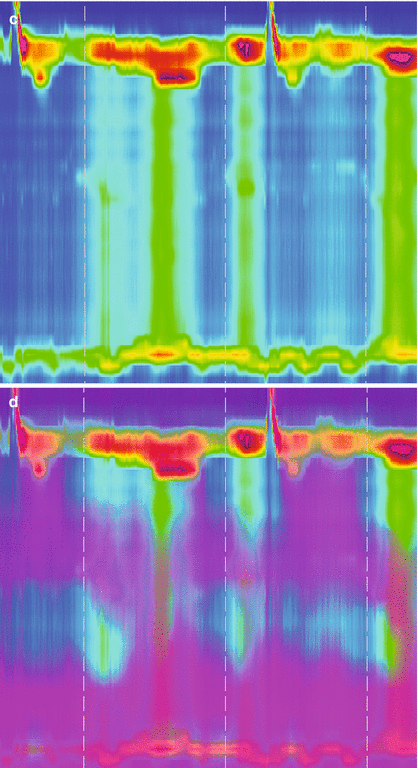


Figure 14.2
High resolution manometry (a, c) with impedance measurement (b, d) in a patient with normal esophageal peristalsis and LES relaxation (a, b) and in a patient with achalasia (c, d). (a) Shows a normal peristalsis and LES. (b) Shows appropriate clearance of the swallowed bolus. (c) Shows absent peristalsis at swallow with no LES relaxation. (d) Shows no clearance of the swallowed bolus
Treatment of Achalasia
Different treatment options for achalasia are available. Several factors should be taken into consideration when determining the appropriate therapy including patient’s age, overall medical condition and the patient’s expectations for symptom relief. Treatment options consist of pharmacologic therapy, endoscopic therapy, and surgical therapy.
Pharmacologic Therapy
The goal of pharmacological therapy is to lower the resting LES pressure. Because drug absorption can be impaired due to the poor esophageal emptying, sublingual medications are preferred. Sublingual calcium channel blockers (nifedipine) and sublingual isorbide dinitrates (nitrates) are the two most common medications used [23, 24]. Less commonly used medications include anticholinergics, beta-blockers, beta-adrenergic agonists, nitroglycerine, and theophylline [25–28].
Improvement of symptoms is reported in 61 % of patients after use of nifedipine and 70 % of patients after use of nitrates [11]. Manometry showed a transient decrease in LES pressure in 46 % of patients, which was better after nitrate use than after nifedipine use. The time to maximum effect was better after nitrates than after nifedipine (25 vs 9 min), but the duration of effect was longer for nifedipine (40 vs 30 min). The short clinical response and common presence of side effects such as headache, dizziness, tachycardia, hypotension, nausea, and ankle edema were limiting problems with pharmacological therapy. It should be considered only for patients who decline or are considered too frail for endoscopic or surgical treatment options.
Endoscopic Therapy
Endoscopic treatments are directed at relieving the obstruction caused by the LES. Standard endoscopic techniques include endoscopic botulinum toxin injection (EBTI) and endoscopic dilation (ED) of the LES. Recently, a novel totally endoscopic esophagomyotomy technique (peroral endoscopic myotomy, POEM) has been introduced in which the endoscope is advanced into an esophageal submucosa tunnel to then divide the inner circular layers of the LES. Currently, no high quality evidence and enough follow up data exist to recommend POEM as a standard therapeutic option for Achalasia, but there are ongoing clinical research trials [29].
Endoscopic Botulinum Toxin Injection (EBTI)
EBTI decreases tonic and swallow-induced LES pressure by inhibiting acetylcholine release from the inhibitory cholinergic presynaptic nerve innervating the LES [30]. EBTI relieves symptoms in 79 % of patients surveyed up to 1 month after treatment, but the symptom relief declines to 70 % at 3 months, 53 % at 6 months, and 41 % after 12 months [1]. Therefore, almost half (47 %) of the patients undergoing EBTI required repeat injection [1]. Relief of dysphagia was found to be better if a second injection was planned at a 1-month interval after the first, but again, symptoms returned in 66 % of patients at 2 years [31]. Primary failure of EBTI can also be due to antibody formation that causes resistance to the acetylcholine injection in 26 % of patients [32–34]. In addition EBTI leads to fibrosis of the mucosa and muscle layers that could make a myotomy, during a future surgical therapy, more challenging [35, 36].
Endoscopic Dilation (ED)
ED attempts to produce a controlled division of the esophageal muscle while leaving the mucosa intact. The current method of choice for dilation is a controlled pneumatic dilation [11]. A balloon is placed across the LES under direct endoscopic or fluoroscopic visualization. The balloon is inflated for 1–3 min, to a pressure of 300 mmHg (10– 12 psi). To obtain an acceptable therapeutic effect, dilation to a diameter of at least 3.0 up to 4.0 cm must be performed; with the 3.0 cm size being preferred as it has a lower perforation rate [37]. Symptom relief seems to dependent on dilator size, the amount of pressure applied and duration of dilation. ED is a relatively safe procedure and the most serious complication of the currently used methods of ED is perforation of the esophagus, which was seen in 1.6 % of patients in a review of 1,065 patients [1]. A systematic review and meta-analysis showed that symptom relief after ED was obtained in 85 % of patients at 1 month and declined with time to 68 % at 12 months and 58 % at 1.5 years [1]. The need for further procedures after ED was 25 % in this review. In addition to dysphagia recurrence, patients undergoing ED can experience the onset of GER [11].
In summary, ED is consistently more durable than EBTI, but after ED, symptoms recur in 42 % of patients over the time and about 30 % of all of the patients treated with ED require further therapy. In a multicenter randomized controlled study [37] clinical outcomes 1 and 2 years after ED were comparable to laparoscopic Heller myotomy with Dor fundoplication. However, patients with ED needed 2 initial dilations with an interval of 1–3 weeks. During the 2 years follow up 4 % did not had a clinical response to initial dilation and additional 27 % had recurrent symptoms with the need for further dilations. Twenty-nine % of the patients with repeated dilations needed an operation. Prior to any surgical intervention, knowledge of the patient’s history of previous endoscopic therapies at the GEJ is important to the surgeon because some experts propose that ED and EBTI lead to fibrosis of the mucosa and muscular layers of the esophagus. Less predictable symptom relief has been reported in patients who have been previously treated with endoscopic therapy [36, 38], which could be due to the greater technical difficulty of doing the operation in these patients.
Peroral Endoscopic Myotomy (POEM)
With this new endoscopic technique (that requires general anesthesia), an esophageal submucosal tunnel through a small opening of the mucosa about 13 cm proximal to the GEJ is created to access the LES and perform a myotomy of the inner circular esophageal muscle LES fibres [29, 39, 40]. Published studies showed good short-term results after POEM with dysphagia remission > 80 % at 12 months [39, 41, 42]. However long-term follow up results are still lacking. Furthermore, results from randomized-controlled trials comparing POEM with other endoscopic techniques or laparoscopic myotomy are not available until now. One major problem after POEM seems to be GER, which has been reported to be up to 46 % [43], because an antireflux procedure cannot be performed simultaneously [29].
Surgical Therapy
The first successful surgical myotomy of the lower esophagus and LES was reported in 1913, by the German surgeon Ernest Heller [44]. His original technique used anterior and posterior myotomies extending for 8 cm or more along the distal esophagus and GEJ through a left thoracoabdominal approach. In 1918, the Dutch surgeon Zaaijer [45] described a modification of Heller’s original technique to a single, anterior cardiomyotomy that has remained the myotomy of choice until now. Both the transabdominal and transthoracic approach have been used to perform a myotomy since. During the end of the last century there was shift from open surgery in the chest and abdomen towards thoracoscopic and laparoscopic surgery. The first laparoscopic Heller myotomy was described by Shimi et al. in 1991 [46]. Thoracoscopic myotomy is more technically challenging and associated with a lower symptom relief but a higher incidence of postoperative GER, making the laparoscopic operation the preferred approach performed at most experienced centers [1, 11]. Laparoscopic myotomy of the LES has proven over the time to be the approach that consistently produces the most durable symptom relief [1, 47].
Some authors have debated the need to perform an antireflux procedure (ARP) after the myotomy [48–50]. A 2009 systematic review and meta-analysis [1] evaluated the development of postoperative GER and found that adding an ARP after laparoscopic myotomy dramatically decreased the incidence of GER symptoms from 31 % down to 9 % (OR 4.3; 95 % CI 1.9–9.7; P = 0.001) without altering the resolution of dysphagia (90 % vs 90 %; OR 1.6; 95 % CI 0.74–3.3; P = 0.23). When measured by 24-h pH monitoring, the incidence of GER after laparoscopic myotomy without fundoplication was 42 % vs 15 % after laparoscopic myotomy with fundoplication (OR 4.2; 95 % CI 1.5–12.8; P = 0.01). The addition of an ARP seems crucial for satisfactory outcome in the treatment of achalasia, and the addition of a fundoplication does not increase morbidity [11, 51].
Laparoscopic Heller Myotomy
Positioning of the patient and Anesthesia
After general anesthesia is induced, the patient is positioned in modified lithotomy and the operating table in reverse Trendelenburg. The patient should be secured to the operating table and all extremities should be padded. The surgeon stands in between the legs of the patient, the first assistant stands on the left side of the patient, and a static retractor (or a second assistant) holds the camera on the right side of the table. In addition to the standard laparoscopic equipment, we also suggest using two graspers with soft grab, scissors, hook cautery, a needle holder, a babcock clamp, a 30° scope, and a 5-mm ultrasonic Harmonic scalpel (Ethicon Endosurgery, Cincinnati OH).
Port Placement
The operation begins with trocar placement similar to that for any laparoscopic operation taking place at the GEJ [47]. Pneumoperitoneum is established to 12 mmHg. Five laparoscopic ports are utilized (three 5 mm and two 10–12 mm). The camera is placed above the umbilicus, one third of the distance to the xiphoid process. In most patients, placement of the camera in the umbilicus will not allow adequate visualization of the hiatal structures once dissected. Two lateral retracting ports are placed in the right and left anterior axillary lines respectively. The port utilized for the liver retractor can be placed at the surgeon’s preference in the sub-xiphoid location or in the right mid abdomen (mid-clavicular line), at or slightly below the camera port. A second retraction port is placed at the level of the umbilicus, in the left anterior axillary line. The surgeons right and left handed trocars are placed in the right and left midclavicular lines, 2–3 inches below the costal margin. Placing the operating trocars on either side of the midline allows triangulation between the camera and the two instruments, avoiding the difficulty associated with the instruments being in direct line with the camera. The falciform ligament hangs low in many patients and provides a barrier around which the left-handed instrument must be manipulated.
Initial Exposure and Dissection
Initial retraction is accomplished with exposure of the esophageal hiatus. A 5 or 10 mm retractor is placed into the sub-xiphoid or right anterior axillary port, and positioned to hold the left lateral segments of the liver towards the anterior abdominal wall. Trauma to the liver should be meticulously avoided, because subsequent bleeding will obscure the field. Mobilization of the left lateral segments by division of the triangular ligament is not necessary. A Babcock clamp is placed into the left anterior axillary port and the stomach retracted toward the patients left foot. This maneuver exposes the esophageal hiatus. An atraumatic clamp should be used, and care taken not to grasp the stomach too vigorously, as gastric perforations can occur. Dissection is typically performed with electrocautery or an ultrasonic dissector. The gastrohepatic ligament is incised to identify and expose the right pillar of the crus. If a replaced left hepatic artery is encountered, it should be preserved. To plan for the extent of the myotomy, the phrenoesophageal membrane is divided and blunt dissection is used to expose the anterior aspect of the abdominal esophagus and the distal portion of the intrathoracic esophagus. This dissection is started by incising just medial to the right crus and dissecting clockwise in a usually avascular plane in between the diaphragm and the esophagus. The anterior vagus nerve should be identified and protected, when possible, to allow the planned myotomy to be performed underneath it (Fig. 14.3).
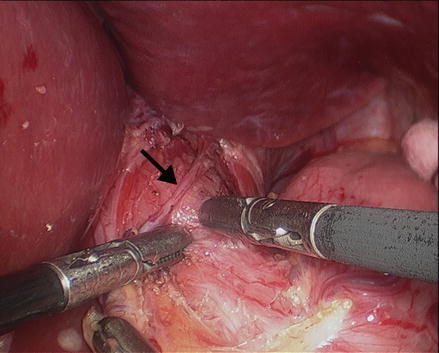

Figure 14.3
Dissection of the phrenoesophageal membrane and the right and left pillar of the crus to expose the anterior aspect of the abdominal esophagus and the distal portion of the intrathoracic esophagus. The anterior vagus nerve (arrow) should be identified and preserved. Two graspers are used to start the myotomy just above the GEJ (Reprinted with permission from Roll et al. [11])
Most specialized centers in the United States choose to add a partial fundoplication after the esophageal myotomy is completed to prevent postoperative GER. Technical details of the construction of an anterior or posterior fundoplication (antireflux procedures) are described below. We routinely perform an anterior (Dor) fundoplication; however, some authors advocate a posterior (Toupet) fundoplication [6, 52, 53]. Anterior fundoplication does not require circumferential esophageal dissection thus allowing for a more limited dissection that leaves the natural adhesions between the posterior esophagus and the hiatus intact, which provides an anchor to help keep the GEJ in the proper anatomic location. If a posterior fundoplication is chosen, the posterior surface of the esophagus and esophageal hiatus must be dissected. In a rare case where a large concomitant hiatal hernia is identified a posterior dissection has to be performed always, so that the hernia can be reduced into the abdominal cavity and the crura can be repaired properly.
Mediastinal mobilization of the esophagus should continue until approximately 6 cm of anterior esophagus is accessible for the myotomy. The anterior fat pad covering the GEJ may be removed to facilitate the myotomy and to better identify the GEJ.
Heller Myotomy
After exposure of the distal esophagus and the GEJ, a myotomy is performed to extend proximally for 6 cm above the GEJ onto the esophagus and distally across the gastroesophageal junction for 3 cm onto the stomach, making the total length of the myotomy about 9 cm. A 48 or 52 French bougie may be placed through the patient’s mouth and through the GEJ into the stomach to help identify the dissection plane and provide tactile feedback during the dissection. The myotomy is started by separating the external longitudinal esophageal muscle fibers on the anterior distal esophagus above the GEJ (Fig. 14.3). This may be done with scissors, a hook, or by tearing the fibers apart using two graspers. There may be bleeding from small vessels, but this is easily controlled with gentle pressure. Excessive use of electrocautery to control bleeding must be avoided at all times, especially if the dissection has traversed the esophageal circular muscle fibers or adhesions and scarring from previous endoscopic treatment are present. The transection of the circular esophageal muscle fibers and the identification of the blood vessels of the submucosa lead to the recognition of the correct dissection plane. Extension of the myotomy from the GEJ onto 3 cm of the proximal anterior stomach to perform a complete myotomy is essential to obtain a successful outcome (Fig. 14.4). A gastric extension that is too short is one important cause of failure of the myotomy to relieve dysphagia. However, most mucosal perforations occur during the extension of the myotomy from the GEJ onto the stomach, because the identification and separation of the muscle layers from the gastric mucosa is more difficult, where the muscularis becomes thinner and more firmly attached to the submucosa. The surgeon should inspect the myotomy area to identify inadvertent mucosal perforations. If a mucosal perforation is detected it must be closed with absorbable interrupted sutures. After completion of the myotomy, a diagnostic upper endoscopy should be performed to ensure that the myotomy is complete, and to insufflate the stomach with the mucosa under water to evaluate for air bubbles indicating a mucosal perforation.
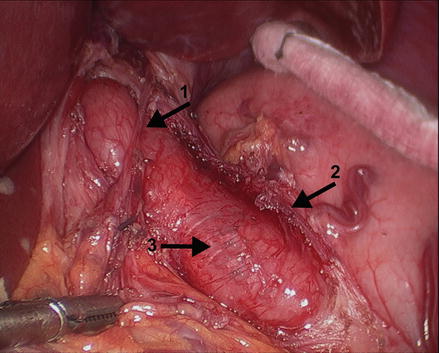

Figure 14.4
The myotomy is performed to extend proximally for 6 cm above the GEJ onto the esophagus and distally across the GEJ for 3 cm onto the stomach, making the total length of the myotomy about 9 cm. Arrow 1: anterior vagus nerve, arrow 2: left edge of the myotomy, arrow 3: exposed esophageal submucosa (Reprinted with permission from Roll et al. [11])
Antireflux Procedure (Fundoplication)
After the myotomy is completed, an antireflux procedure is performed to prevent postoperative GER by recreating the His angle and keeping the GEJ inside the abdominal cavity. A 360° Nissen fundoplication has been used in selected series [51, 54, 55], however, a Nissen fundoplication may hinder esophageal clearance, resulting in progressive postoperative dilatation of the aperistaltic esophagus and recurrent dysphagia. Although a few centers with significant experience in esophageal surgery still advocate a Nissen fundoplication after myotomy [55], most do not recommend it due to reported reoperation rates as high as 29 % [11]. Two partial fundoplications have been used with equipoise, a posterior fundoplication (Toupet fundoplication) and an anterior fundoplication (Dor fundoplication). The theoretical advantages of the Toupet fundoplication are that due to its anatomical configuration, it keeps the edges of the myotomy pulled apart, thus preventing scarring and recurrent dysphagia, and that it can be performed just after the lower esophagus has been pulled downward and straightened, thus improving passage through the cardia and again minimizing postoperative dysphagia [56]. The drawbacks of the Toupet fundoplication are the need for circumferential dissection of the GEJ and the possibility that diverticula may develop at the site of the myotomy years after surgery because the fundoplication does not cover the myotomy site [57]. Proponents of the Dor fundoplication argue that the procedure is faster because the posterior esophageal attachments may be left in place [56]. Another advantage is that a properly constructed Dor fundoplication can prevent post-operative reapproximation of the myotomy [58]. Furthermore, covering the myotomy with the fundoplication may seal inadvertent mucosal injury and prevent future development of diverticulae at the site of the myotomy.

Full access? Get Clinical Tree








