(1)
Botulinum Toxin Treatment Program, Yale School of Medicine, New Haven, CT, USA
Abstract
Primary headaches consist of migraine, tension-type headaches, and trigeminal autonomic cephalalgias (e.g., cluster headache). Migraine and tension-type headaches are common and, in chronic form, are major causes of disability. Prospective double-blind and placebo-controlled studies have confirmed the efficacy of onabotulinumtoxinA (onaA) in chronic migraine (class A evidence). Follow-up of patients in PREEMPT studies with five cycles of onaA injections (over 56 weeks) attests to the tolerability and safety of this drug in chronic migraine and demonstrates progressive improvement of patient’s quality of life. In clinical practice, patients treated with onaA for more than 2 years describe their experience as very gratifying (attached videotapes). Studies using onaA in management of episodic migraine and chronic daily headaches have shown disappointing results and no evidence of efficacy. Investigations using botulinum neurotoxins (BoNTs) in management of tension-type headache have also provided negative results, but the results are confounded by selection of low-dose, suboptimal technique and selection of rigid primary outcome criteria. No blinded studies with BoNTs are available for trigeminal autonomic cephalalgias.
Electronic supplementary material
The online version of this chapter (10.1007/978-1-4939-2501-8_4) contains supplementary material, which is available to authorized users.
Keywords
Episodic migraineChronic migraineTension headacheChronic daily headacheAutonomic neuralgiasCluster headacheBotulinum toxinBotulinum neurotoxinOnabotulinumtoxinAOnaAIntroduction
Headache is a common human ailment with an annual prevalence of 90 % and lifetime prevalence of 99 % (Evans 2005). The Headache Classification Subcommittee of the International Headache Society (IHS) has classified headaches into primary and secondary categories (Headache Classification Subcommittee of HIS 2004, 2nd edition). The focus of this chapter is on primary headaches (migraine, tension headache, and trigeminal autonomic cephalalgias: cluster and others) for which data on BoNT efficacy is available.
Migraine
Introduction
Migraine is a common primary headache disorder that affects 18 % of women and 6 % of men (Lipton and Silberstein 2001). It is the most complex form of human headache due to the great variability of its symptoms. Migraine is a major financial burden to the society with an estimated annual cost of $4.2 billion for direct cost of care in the USA (Insinga et al. 2011).
Migraine headache is subclassified into migraine with aura (MWA) and migraine without aura (MWoA). The pain is characteristically pulsating, starts often unilaterally, and is frequently associated with photophobia, phonophobia, and gastrointestinal distress. Migraine’s aura often involves the visual system with either enhancement of function (bright or zigzag lights) or loss of function (scotoma). Loss of function may also occur in the motor (hemiplegic migraine) or other sensory domains. Local scalp tenderness and allodynia (touch perceived as pain) are common in migraine and were reported to affect 43 % of 89 patients in one study (Ashkenazi et al. 2007). In another study, the incidence increased with the number of migraine attacks, 33 % associated with one to four attacks and 58 % associated with more than eight attacks/month (Mathew 2011). Allodynia usually starts ipsilateral to the side of headache, denoting activation of peripheral nociceptive pathways. Contralateral spread of allodynia indicates central sensitization to pain via third-order (thalamic) neurons (Burstein et al. 2000).
In episodic migraine, attacks occur less than 15 days per month. Chronic migraine is defined as headaches occurring 15 or more days per month, lasting more than 3 months with 8 or more days per month meeting the criteria for migraine without aura or responding to migraine-specific treatment (Headache Classification Subcommittee of HIS 2004). Chronic migraine includes approximately half of all chronic daily headaches and has an estimated global prevalence of 2 % (Natoli et al. 2010). It is the most costly form of migraine with nearly $200 per week more cost to the employers than episodic migraine (Serrano et al. 2013).
Pathophysiology
The aura phase of migraine corresponds to the electrical phenomenon of cortical spreading depression (SD) that often involves the occipital cortex (predominance of visual aura) (Lance and Goadsby 2005). Spreading depression marches through the cortex at the rate of 3–6 mm/min does not respect specific vascular territories (Cao et al. 1999). What triggers and initiates SD has remained elusive. It is believed that the release of extracellular potassium, nitric oxide, adenosine, and others agents during cortical depression causes inflammation and vasodilation in the cortex and meningeal vessels (Waeber and Moskowitz 2003). This results in a sensitized trigeminovascular system which sends enhanced afferent impulses to the trigeminal ganglion, nucleus pontis caudalis, superior salivatory nucleus, and parasympathetic efferent fibers (Noseda and Burstein 2013). Excitation of the latter causes dural vasodilation. Emergence of nociceptive stimuli at different levels of the nervous system and the trigeminal nucleus causes head and facial pain.
The genetics of migraine was reviewed in a recent communication (Silberstein and Dodick 2013). First-degree relatives of patients with MWA and MWoA have 4 and 1.9 times the risk of developing the same type of migraine, respectively. Approximately, 52 % of female twins raised together or apart demonstrate co-occurrence. Aside from familial hemiplegic migraine which is monogenic (with three identified genes), the genetics of other forms of migraine is complex, and the information is still preliminary and evolving. Recently, mutation of CNK18 gene with complete loss of function of related K channel was detected in some patients with MWA (Lafreniere et al. 2010).
Reliable biomarkers for diagnosis of migraine are often sought. A recent communication reported that serum level of CGRP is 2.5 times higher in CM compared to asymptomatic controls and about 1.8 times higher than patients with episodic migraine or cluster headaches (p < 0.05) (Cernuda-Morollon et al. 2013).
Treatment
Treatment of migraine headaches includes strategies to abort acute attacks and, in case of frequent attacks, reduce the frequency of attacks by daily medications (preventive drugs). Several recent comprehensive reviews have discussed treatment strategies for migraine (Silberstien 2008; Mathew 2011; Diener et al. 2011). Treatment of mild migraine attacks includes the use of acetaminophen, aspirin, and nonsteroidal anti-inflammatory drugs (NSAIDs). For more severe attacks not responding to these measures, triptans are often recommended. Triptans act on 5HT receptors in the trigeminal nucleus caudalis and in the dorsal horns of the upper cervical spine, hence interfering with the nociceptive cascade beginning to set in the trigeminovascular system (Goadsby and Knight 1997). Many patients with migraine, however, do not respond to triptans, and cardiovascular comorbidities often limit their use (Hoffmann and Goadsby 2014). For attacks refractory to oral medications, liberal hydration (IV fluids) and intravenous administration of dopamine receptor agonists (prochlorperazine), dihydroergotamine (DHE), or IV NSAIDs (diclofenac or ketorolac) are recommended (Gelfand and Goadsby 2012). One small study has shown that high flow oxygen may alleviate acute attacks of migraine (Ozkurt et al. 2012). Opiates, barbiturates, and short course of steroids are also used as abortive therapy by some clinicians, but supportive studies are lacking. Since most abortive medications are associated with significant comorbidities, development of effective preventive drugs with safe profile is urgently needed.
Preventive daily treatment of migraine is recommended when migraine episodes exceed six to eight headache days per month or if the patient has to use abortive medications more than eight to nine times per month (Silberstein 1997). Beta-blockers such as propranolol or metoprolol, topiramate, gabapentin, amitriptyline, and sodium valproate are commonly used for migraine prevention (Goadsby 2013). Venlafaxine and histamine are considered second-line preventive medicine.
For chronic migraine, double-blind studies are available on the efficacy of topiramate (two large multicenter studies; Lanteri-Minet et al. 2007; Diener et al. 2007), valproate, and levetiracetam (both small studies). Both double-blind multicenter US and European studies have demonstrated significant reduction of headache days and migraine days per month with topiramate (in the USA study, 3 days reduction with topiramate vs. 0.7 days with placebo). The effective dose was 100 mg/day. The double-blind/parallel valproate study (Yurekli et al. 2008) and the double-blind/crossover levetiracetam study (Beran and Spira 2011) encompassed 41 and 71 patients, respectively. Both studies showed effective reduction of headache days per month and headache severity in the BoNT group versus placebo. For levetiracetam, the primary endpoint—absence of any headaches—was not met, however, perhaps due to the rigidity of the criteria.
Botulinum Neurotoxin for Preventive Treatment of Migraine
Episodic Migraine
The first double-blind, placebo-controlled, prospective study (class II) investigating efficacy of onabotulinumtoxinA (onaA) in episodic migraine was published in 2000 (Silberstein et al. 2000). The authors investigated the effect of 25 and 75 units of onaA in 123 patients with two to eight migraine attacks per month. Patients with headache days exceeding 15/month (chronic migraine) were excluded. OnaA was injected into the procerus muscle (3 or 9 units) and bilaterally into corrugators (two on each side, 6 or 18 units), frontalis (two on each side, 6 or 8 units), and temporalis (one on each side, 6 or 18 units) muscles.
Primary efficacy was defined as a significant change from the baseline of migraine attacks. At 3 months, patients in the 25 unit group had significant reduction in headache frequency and headache intensity and 50 % reduction of headaches compared to baseline. No statistically significant change was noted in the 75 unit group, a finding attributed to their milder headaches at baseline. Subsequently, two large class I studies were conducted with onaA in EM investigating 238 and 418 patients (Elkind et al. 2006; Saper et al. 2007). Both studies failed to meet their primary outcome measure that was reduction of migraine frequency/month. Another small (60 patient) class II study of EM that considered 50 % or more reduction of migraine frequency as the primary outcome also failed to meet its primary endpoint (Evers et al. 2004). The total dose applied in the aforementioned studies varied from 25 to 100 units. The American Academy of Neurology’s subcommittee on guidelines based on the above four studies (two class I and two class II) assigned level B evidence (probably ineffective) to onaA for the treatment of episodic migraine (Naumann et al. 2008).
Two other class I studies which were published later and used larger doses of onaA confirmed the stance of AAN’s subcommittee on episodic migraine (Relja et al. 2007; Aurora et al. 2007). The first study compared the effect of different doses of onaA (75, 150, 225 units) with placebo using the mean number of migraine days at day 180 as the primary outcome measure. All four groups (including the placebo group) improved with either onaA or saline (the placebo), and there was no significant difference between onaA subgroups and the placebo group (Relja et al. 2007). In the second study of 369 patients (Aurora et al. 2007), the authors compared the effect of onaA (mean 190.5 units) with placebo. The primary endpoint, defined as the mean change in migraine episodes over 30 days prior to day 180, was not met. Although the study failed to meet the primary endpoint, a subgroup analysis of patients with 12–14 headache days per month showed significant improvement in onaA group versus placebo (p = 0.04).
Chronic Migraine
In a small study, Freitag et al. (2008) compared the effect of fixed-dose (100 units) and fixed-site (glabella, frontalis, temporalis, trapezius, suboccipital) injections between onaA (20 patients) and placebo (21 patients) and reported promising results for chronic migraine. Patients with medication overuse were excluded. The primary outcome was the number of migraine episodes with each 4 weeks of the study. The secondary outcomes included the number of headache days and the headache index (a measure of both intensity and frequency). OnaA was statistically superior to placebo on both primary outcome (p < 0.01) and secondary outcomes (p = 0.041 and p = 0.046) and for headache index (HI) at 16 weeks (p = 0.003). Nevertheless, between 2002 and 2009, a number of large multicenter studies assessing efficacy of BoNTs in chronic migraine failed to meet their primary outcome. The major breakthrough in this area came with the publication of PREEMPT studies (I and II) in the summer of 2010 (Aurora et al. 2010; Diener et al. 2010). Each of these two multicenter studies evaluated approximately 700 patients (total 1,384 patients) who met the criteria for chronic migraine. Patients with medication overuse were included in both studies. The studies had a 24-week blinded arm followed by 32 weeks of open arm. The primary outcome for PREEMPT I was the number of migraine episodes and for PREEMPT II, the number of headache days, both evaluated at 24 weeks. A number of secondary outcomes were also evaluated at the 24-week time point. PREEMPT II met both its primary and secondary outcomes at all time points (Fig. 4.1). For the primary outcome, the change in headache days was 9 for onabotulinumtoxinA versus 6.7 for the placebo (p < 0.001). Although PREEMPT I did not meet its primary outcome, it met all its secondary outcomes. The pooled data (Dodick et al. 2010) from the two studies showed significant change from the baseline in favor of onabotulinumtoxinA in respect to the primary and all secondary parameters. Based on these studies, onabotulinumtoxinA was approved for the treatment of chronic migraine in the UK, Canada (summer of 2010), and in the USA (October 2010).
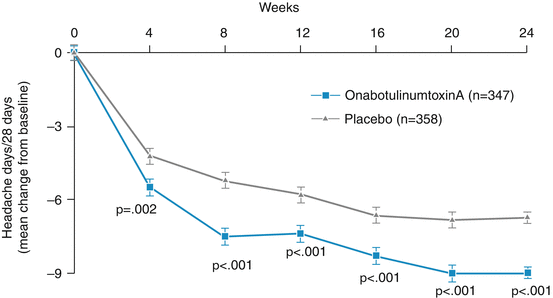

Fig. 4.1
Significant reduction in pain days from botulinum toxin group compared with placebo group over all time points of 24-month blinded arm of the study (From Diener et al. (2010). © 2010 SAGE Publications. Reprinted with permission from SAGE)
Subsequently, Lipton et al. (2011) studied the PREEMPT pooled data (1,384 patients) specifically in regard to the quality of life which was measured by both the Migraine-Specific Quality of Life Questionnaire (MSQ) and the Headache Impact Test (HIT). Both measures were significantly improved from the baseline in the onaA-treated group at 24 weeks providing strong evidence for quality of life improvement with onaA in chronic migraine.
BoNT Injection Technique in Chronic Migraine
A variety of injection techniques have been proposed for BoNT treatment of chronic migraine based on established studies and the practice of experienced BoNT practitioners. The technique used in PREEMPT studies (Blumenfeld et al. 2010), recommends 31 injection sites, a total dose of 155 units, and injecting of 5 units per site (Fig. 4.2). In some patients, an additional 40 units is allowed for a total of 195 units. A dilution of 100 units/2 cc was recommended. Silberstein from Jefferson’s comprehensive headache clinic in Philadelphia endorses a technique similar to PREEMPT with more injection points over the frontal region (Silberstein et al. 2013).
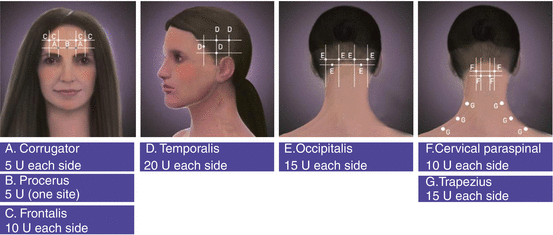

Fig. 4.2
Injection sites for treatment of chronic migraine based on PREEMPT studies (From Blumenfeld et al. (2010). © 2010 American Headache Society. Reprinted with permission from John Wiley and Sons)
Over the past 15 years, I have used an injection scheme at Yale which provides a high rate of success (nearly 90 %) in chronic migraine (Fig. 4.3). The total number of injections is 23, and the total dose is 165 units. In patients with large necks, an additional 30 units is injected into the cervical paraspinal muscles. In this technique, on each side, 5 units are injected into the corrugator muscle, 15 units into the frontalis muscle (5 units/site), 30 units into temporalis (15 units anterior and 15 mid-temporal), 10 units into occipitalis (1/site), and 30 units into the splenius muscles (10 units per site). An additional 5 units is injected at midline into the procerus muscle. The injection pattern emphasizes the importance of temporalis and posterior neck muscles in chronic migraine and does not include horizontal, shoulder trapezius muscles. Although Ashkenazi and Blumenfeld (2013) warn against development of weakness in cervical and trapezius muscles if the dose per injection site exceeds 5 units, I have never seen weakness of these muscles following 10 units/site injection. This is probably because cervical paraspinal and trapezius muscles are very strong and multilayered muscles. Our technique has also the advantage of applying fewer injections of 23 versus 31 employed in PREEMPT studies.
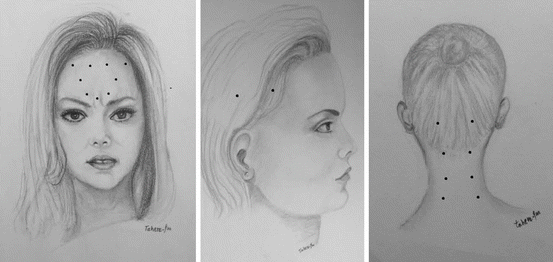

Fig. 4.3
Injection sites and doses of onaA for chronic migraine per author’s protocol at Yale. The emphasis is on fewer number of injections, higher dose in anterior temporal and posterior cervical muscles (Created by Tahereh Mousavi; published with kind permission from © Bahman Jabbari 2014. All Rights Reserved)
The Issue of Medication Overuse in Chronic Migraine
Medication overuse is a common problem as many patients with chronic migraine have medication overuse problems. Inclusion of patients with medication overuse headaches in PREEMPT studies has raised questions by some investigators. Silberstein et al. (2013) studied the efficacy of onaA in a subgroup of PREEMPT study patients who had medication overuse in addition to chronic migraine (MO + CM). Of 1,384 patients in the PREEMPT study, 65.3 % met the criteria for medication overuse. At 24 weeks, similar to the patients in the main PREEMPT study, MO + CM patients demonstrated significant improvement of headache days (primary end point) (−8.2 vs. −6.2 with p < 0.001) and also met many secondary endpoints (frequency of migraine days, frequency of moderate to severe headache days, cumulative headache hours on headache days, headache episodes, migraine episodes, and percentage of patients with severe HIT-6 (all Ps < 0.05). Triptan intake was also significantly reduced in the onaA-treated group (p < 0.001). Authors concluded that onaA treatment is effective in patients with chronic migraine with MO.
Sandrini et al. (2011) studied the effect of onaA injections in 68 patients with chronic migraine without aura and with MO (35 placebo, 33 toxin). The study was double blind and placebo controlled with primary and secondary outcomes measured at 12 weeks. Patients received a total of 16 injections (100 units), 8 on each side (2 frontal, 2 cervical, 1 corrugator, 1 temporal, 2 trapezius). No significant difference was noted between the placebo and toxin in reduction of pain days (primary outcome). A subgroup analysis of the data, however, demonstrated that MO patients with pericranial tenderness had significantly lower number of pain days (primary outcome). The total dose of 100 units used in this study was probably too small; also, some important head regions (occipital) were not covered.
Long-Term Response to BoNTs in Chronic Migraine and Safety Issues
Although many BoNT practitioners with substantial experience in the treatment of migraine have long believed in the long-term efficacy and safety of onaA, until recently, no systematic data was available. In 2014, Aurora et al. published data on safety, tolerability, and efficacy of onaA (PREEMPT study) after five cycles of treatment (at 56 weeks). The mean change in frequency from baseline of headache days, migraine days, and moderate to severe headache days and 50 % or more change in headache days from baseline were all significantly lower (p < 0.05) in the onaA treatment group. The quality of life was further improved at 56 weeks (59 %) compared to 25 weeks (44 %), measured by 5 or more points increase in the HIT-6. No cumulative undesirable effects were noted. Tolerability was excellent, and there were no serious safety issues.
Personally, I have been very impressed with the long-term efficacy of onaA in chronic migraine. A prospective observation (unpublished data) of my last 80 patients with over 2 years follow-up and treated with the methodology illustrated in Fig. 4.3, excluding cases of litigations and secondary gain, disclosed an efficacy of 90 %. In general, the second and third injections were more effective than the first. At least 80 % of the patients reported significant improvement in their quality of life (attached videos). Side effects were uncommon and included transient local bleeding, local neck pain, and transient frontal asymmetry including the Mephisto sign. The Mephisto sign refers to an elevation of the lateral part of the eyebrow (Fig. 4.4). Cho et al. (2013) recommend an additional, lateral frontal injection to avoid elevation of the lateral part of the eyebrow. I have not seen any serious side effects with onaA treatment of chronic migraine with over 3,000 injection sessions.
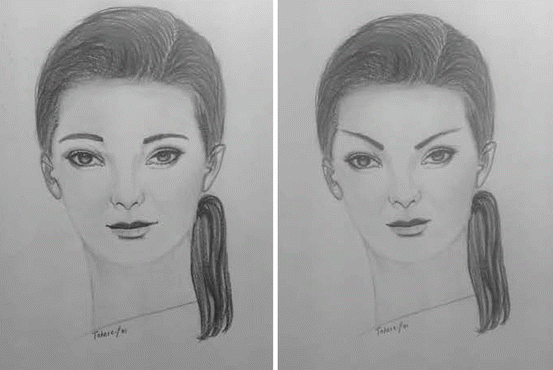
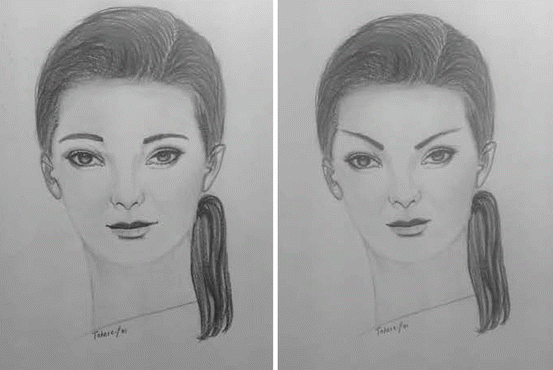
Fig. 4.4
Mephisto sign, elevation of the lateral part of eyebrows following injection of frontalis muscles in migraine. This finding is attributed to injections affecting lateral and medial brow elevators unequally (Created by Tahereh Mousavi; published with kind permission from © Bahman Jabbari 2014. All Rights Reserved)
Imploding Versus Exploding Migraine
In a study of 63 patients with migraine, Jakubowski et al. (2006) found patients with imploding headaches to be better responders to onaA than those with exploding headaches. Among responders to onaA, 74 % had imploding headaches; among nonresponders, 92 % had exploding headaches. Imploding headaches were described as those with pressure from outside the head (crushed, clamped, or stabbed by an external force). Exploding headaches were headaches felt as pressure built inside of the head. This is an interesting and perhaps an important concept. Distinction between exploding and imploding headaches is not always easy in patients with chronic migraine and requires focused questioning by examining physicians.
Patient 4-1
A 32-year-old female complained of frequent migraine attacks for 7 years. The attacks gradually increased in frequency despite taking triptans for acute attacks and a number of preventive medications. The last preventive drug before BoNT treatment was topiramate (100 mg daily). Patient had daily headaches with three to four severe migraine episodes per week. During the episodes, she had pounding/pulsating headaches with nausea and photophobia. She stopped working 2 years ago due to disabling headaches. After the second session of botulinum toxin treatment with onaA, the patient reported significant reduction in frequency and intensity of the headaches that improved even further with subsequent treatments.
A year after initiation of treatment with onaA, she was able to stop all preventive medications. Six months later, she resumed her work and became gainfully employed. At 2 years posttreatment evaluation, she reported few headaches, was fully functional, and expressed relief (Video 4.1). Video 4.2 shows her at 2 years post-onaA treatment during an injection session (using the PREEMPT scheme). Video 4.3 shows an injection session in a male patient with 3 years of onaA treatment and a 10-year history of debilitating migraine. Video 4.4 shows this patient’s interview, 3 years after onaA treatment for chronic migraine. Videos 4.5 and 4.6 demonstrate our injection technique in a female patient with chronic migraine and an interview with the patient.
The Mechanism of Action of onaA in Chronic Migraine
Animal studies have demonstrated an analgesic effect for botulinum toxins via different mechanisms (see Chap. 2). These mechanisms include inhibition of the release of pain mediators (calcitonin gene-related peptide, glutamate, and substance P) from peripheral nerve endings and dorsal root ganglia (Aoki 2005; Meng et al. 2007; Lucioni et al. 2008). Furthermore, onaA reduces tissue inflammation and local accumulation of glutamate in the formalin model of pain (Cui et al. 2004). Peripheraly injected onaA has been shown to travel to sensory ganglia, and there is some evidence that it may reach central nervous system from the site of peripheral injection (Wiegand et al. 1976). In animals, experimentally induced spreading depression releases pain mediators and pro-inflammatory agents in meningeal and dural nerve endings; the same is probably the case in migraine (Noseda and Burstein 2013). Direct excitatory cortical connections to neurons of trigeminal nucleus and brain stem nuclei also lead to inflammatory changes and increased release of pain mediators (especially CGRP). These changes lead to peripheral and central sensitization of the neural tissue (Aoki 2005).
It has been postulated that after injection, onaA reaches trigeminal and cervical nerve endings locally. The relief of migraine is via blocking the release of aforementioned pain mediators and reduction of peripheral and central sensitization (Ashkenazi and Blumenfeld 2013).
The effect of onaA on pericranial muscles may also partially explain its effect on chronic migraine. In CM, pericranial muscles are often tense and display increased tone. In tense muscles, intrafusal muscle fibers (muscle spindles) have a higher discharge rate. Increased non-nociceptive input to the central nervous system can worsen central sensitization as in this state, non-nociceptive input can be perceived as nociceptive by wide range function neurons (Roberts 1986). Muscle spindles are a major source of non-nociceptive input to the central nervous system. In animals, injection of onaA into the muscle markedly reduces the discharge of muscle spindles (Filippi et al. 1993).

Full access? Get Clinical Tree








