Mechanical Ventilation during General Anesthesia: Introduction
In the early years following the first demonstration of anesthesia with an inhalation device, different inhalation anesthetic agents were developed and tested in spontaneously breathing subjects. However, the increasing complexity of surgical procedures, the discovery and use of neuromuscular blocking as well as intravenous anesthetic agents, and the need to protect the airways resulted in the need for mechanical ventilation during general anesthesia. Accordingly, anesthesia ventilators were developed to match the particular needs of general anesthesia, which differ from those in other settings, such as the intensive care unit (ICU). Moreover, modern anesthesia is not restricted to the intraoperative period and mechanical ventilation may be required preoperatively and postoperatively.
Respiratory Effects of Anesthesia and Surgery
General anesthesia has several effects on respiratory function, most notably on control of breathing and the activity of respiratory muscles, which influence the distributions of ventilation and perfusion within the lungs. Moreover, surgery-related positioning of patients, as well as manipulation or displacement of intraabdominal and thoracic organs may further affect ventilation and perfusion, with deterioration in pulmonary gas exchange.
The main objective of control of breathing is the maintenance of blood gases, especially of arterial carbon dioxide (PaCO2), which is kept within a relatively restricted range. Even a small increase in PaCO2 may result in a significant increase in minute ventilation. The output of the respiratory center is less sensitive to reductions in partial pressure of arterial oxygen (PaO2), but hypoxia may enhance the response to PaCO2.1 Another objective of the control of breathing is to maintain the brain pH. Because CO2 is highly diffusible across the blood–brain barrier, this is achieved through modulation of alveolar ventilation, which regulates PaCO2. Last but not least, control of breathing aims at optimizing breathing frequencies and maximizing the work output of the respiratory muscles to maintain adequate gas exchange.2
The respiratory rhythm originates from complex neuronal network interactions throughout the nervous system.3 This network consists of smaller and larger networks that interact in generating different breathing patterns, including the regular breathing (eupnea), but also sighs and gasping. The group of neurons responsible for the control of breathing is dispersed across a region in the brainstem termed the respiratory centers. These centers are located bilaterally in the reticular formation of the medulla oblongata and pons, beneath the floor of the fourth ventricle, and are grouped into inspiratory, pneumotaxic, and expiratory areas.4
The inspiratory area is located in the dorsal part of the medulla and generates rhythmic cycles that are transmitted to the diaphragm, upon contraction of which alveolar ventilation takes place. Impulses originating in peripheral and central chemoreceptors (e.g., in carotid bodies and in the brainstem, respectively), and lungs are transmitted through the vagus and glossopharyngeal nerves to the inspiratory area, downregulating its activity in a negative-feedback mechanism.5 Impulses from the pneumotaxic center located in the pons inhibit the inspiratory area continuously, contributing to avoidance of overinflation and modulation of respiratory rate.6 Normally, exhalation is a passive process resulting from the elastic recoil of the lungs and chest wall, and the expiratory center located in the ventral portion of the medulla is silent. This center, however, may be activated for increased alveolar ventilation, leading to recruitment of thoracic and abdominal muscles and forced expiration. In addition, alveolar ventilation is regulated by the cerebral motor cortex during voluntary control of breathing, and impulses bypass the respiratory centers being transmitted directly through corticospinal to respiratory motoneurons.7
Most drugs used to achieve hypnosis, sedation, and relief or suppression of the response to pain affect the control of breathing. Such drugs, which usually result in depression of the alveolar ventilation, have their effects mediated by direct interference with peripheral and central chemoreceptors, changes in behavioral control, suppression of the function of the motoneurons and respiratory muscles, and by general depression of the respiratory center.8
Volatile halogenated anesthetic drugs like halothane, isoflurane, enflurane, desflurane, and sevoflurane reduce the response of the respiratory center to hypoxia even at subanesthetic concentrations.9–13 In addition, volatile anesthetics can potentiate the effects of neuromuscular blocking agents on the respiratory muscles in a dose-dependent and time-dependent fashion.14 Intravenous anesthetic drugs like midazolam and propofol also blunt the response to hypoxia at subhypnotic concentrations,15,16 while hypnotic concentrations of barbiturates are necessary for decreasing the ventilatory response to hypoxia.17 Although anesthetics usually depress the respiratory center activity, subhypnotic concentrations of etomidate stimulate ventilation,18 while during infusion of ketamine, the response to hypercapnia is fairly well maintained19 or even increased.20
Opioids represent a mainline therapy for analgesia and anesthesia. These drugs, however, can reduce and make the respiratory rate irregular,21 resulting both in hypercapnia and hypoxemia. Such effects are mediated mainly by opioid receptors expressed in the peripheral chemoreceptors.22 When infused rapidly as boluses, opioids have the potential to abolish the respiratory center activity completely without producing unconsciousness. Furthermore, opioids can increase the resistance of the upper airway by enhancing the discharge activity in the recurrent laryngeal nerve, and reduce the compliance of the respiratory system through increases in tonic discharges from the expiratory area of the respiratory center.23
Even when administered in the intrathecal or epidural space, or intramuscularly, opioids have the potential to induce respiratory depression in approximately 1% of patients.24 Respiratory depression induced by epidural opioids results either from systemic absorption, when delayed, or from cephalad migration through the cerebrospinal fluid, when it immediately follows the administration of the drug. Factors that magnify respiratory depression following intrathecal and epidural opioids are: (a) use of intravenous anesthetic drugs; (b) coughing, which may contribute to spread of the drug within the cerebrospinal fluid; (c) accumulation through higher and/or repeated doses; (d) use of morphine; (e) advanced age; (f) lack of opioid tolerance; (g) thoracic epidural placement; (h) general anesthesia; and (i) increased thoracic pressure.24
Depression of inspiratory muscle activity during anesthesia occurs not in a global but rather in a selective manner. The intravenous administration of the barbiturate thiopentone decreases the mean activity of the genioglossus, sternothyroid and sternohyoid (strap muscles), and scalene muscles in patients.25 Furthermore, tonic pattern of activity of the strap and scalene muscles disappears and those muscles become synchronized with inspiration.
Although diaphragmatic activity is fairly well preserved, the intercostal muscles become silent at concentrations between 0.2 and 1.0 of the minimal alveolar concentration.26,27 As a result, a pattern of breathing originates where diaphragmatic activity expands the lower ribcage and the resulting negative intrathoracic pressure moves the upper ribcage inwards. Such pattern can be even more pronounced if the resistance of the upper airways is increased, for example, when narrow endotracheal tubes are used. In fact, it is conceivable that increased resistance solely determines the limited expansion of the ribcage during inspiration. It has been namely shown that one minimal alveolar concentration isoflurane does not reduce ribcage motion, unless hyperpnea occurs.28 Accordingly, ribcage motion during inspiration is not depressed by anesthesia with ketamine.29
In contrast to the inspiratory muscles, the expiratory muscles are usually inactive in awake subjects, becoming active during anesthesia. In patients anesthetized with halothane, Warner et al26 attributed active expiration to the motion of the internal intercostal muscles. In fact, most anesthetic agents have been implicated with active expiratory muscle activity.
The purpose of active expiration is not well determined, and its contribution in reducing functional residual capacity (FRC) during anesthesia is controversial.30,31
Reductions in FRC of as much as 20% during general anesthesia have been reported in healthy individuals and can be larger in the presence of pulmonary comorbidities and obesity.32 Such deterioration occurs early after induction of anesthesia and is stable over time.33 Also, spontaneous breathing or increases in airway pressure cannot easily reverse a reduced FRC, which persists for hours after recovery from anesthesia.32 Various mechanisms explain the reduction of FRC during anesthesia: (a) changes in the chest wall; (b) changes in the diaphragmatic shape and position; and (c) increase in thoracic blood volume.
The loss of inspiratory muscle tone associated with anesthesia results in reduced outward recoil of the chest wall, and of the internal diameters of the ribcage,32 leading to changes of approximately 200 to 300 mL in lung volume.34–37 In addition, an increase in the anterior curvature of the border of vertebral bodies consistent with increased spinal curvature has been detected.37
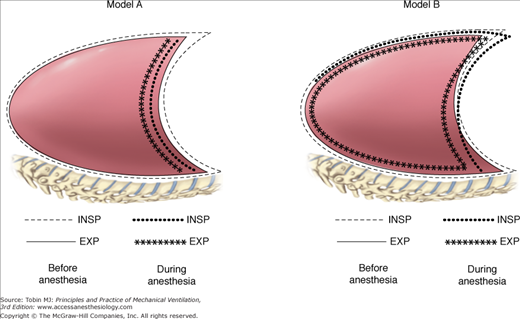
Loss of muscle tone during induction of anesthesia may favor a cephalad movement of the diaphragm secondary to pressure of the intraabdominal organs. Although such a mechanism hypothesis has been supported by some authors,34,38 it has been challenged by others. Using a dynamic spatial reconstructor technique, Warner et al31 showed that it is not the position, but the shape of the diaphragm, that is altered during anesthesia. They31 postulated that the most dorsal part of the diaphragm shifts cephalad, while the most ventral part shifts only minimally, or even caudally. Figure 24-1 schematically illustrates the concepts of change in diaphragmatic position and shape.
Figure 24-1
Models of chest wall and diaphragmatic displacement during general anesthesia. In model A, a cephalad shift of the diaphragm is postulated;34 in model B, the displacement of the ventral part of the diaphragm is caudal, while the dorsal part shifts cranially.31 Both models are associated with a decrease in functional residual capacity (FRC). Exp, expiration; Insp, inspiration.
Increases in intrathoracic blood volume resulting from redistribution of blood from the periphery could theoretically further reduce FRC, although this mechanism is controversial.34,39
The concept of atelectasis formation during general anesthesia was first proposed by Bendixen et al40 in 1963 to explain a progressive decrease in oxygenation and compliance. Those authors, however, could not confirm that hypothesis using chest radiographs. In addition, other authors were not able to reproduce those findings, observing a prompt rather than gradual change in respiratory mechanics under anesthesia.33
Collapse of lung zones during anesthesia was first confirmed using computed tomography,41 and it has been estimated that 90% of patients develop atelectasis under general anesthesia.42 Several mechanisms have been suggested to explain the formation of atelectasis during anesthesia: (a) collapse of small airways; (b) compression of lung structures; (c) absorption of intraalveolar gas content; and (d) impairment of lung surfactant function. These mechanisms are not mutually exclusive and may interact with each other.
A reduction in FRC below the closing capacity during anesthesia can result in collapse of lung units.43 Two studies documented a decrease of closing capacity and FRC in anesthetized patients,44,45 but this finding is not indisputable.46 In the presence of increased smooth muscle tone of the small airways, collapse can occur even at FRC values above the closing capacity, for instance in patients with chronic obstructive pulmonary disease (COPD) and asthma, or those receiving anesthetic drugs that promote histamine release and cause bronchoconstriction (e.g., thiopental, atracurium, d-tubocurarine).
In the supine position, there is a ventral–dorsal gradient in the transpulmonary pressure.47,48 This gradient is determined by the shape of the chest wall and lungs, as well as by lung height multiplied by the tissue density, which may promote atelectasis. Similarly, because the lung area under the heart is greater in the supine than in the prone position,49 the weight of the heart could theoretically favor atelectasis during general anesthesia. Furthermore, the pressure of intraabdominal organs can be transmitted in part to the lung structures in the absence or reduction of diaphragmatic muscle tone.50 In addition, increases in intrathoracic blood volume resulting from redistribution of blood from the periphery could theoretically further reduce FRC but this mechanism is controversial.34,39
In the presence of an increased ventilation-to-perfusion ratio, high alveolar oxygen concentrations can lead to a progressive loss of alveolar total gas volume. A computed tomography study showed that 16% to 20% of lung tissue develops collapse or is poorly aerated in patients under general anesthesia.51 Absorption atelectasis is most relevant when small airway closure occurs, as for instance following reduction of the FRC beyond the closing capacity. The pulmonary circulation then takes up the trapped gas enriched with oxygen, resulting in atelectasis. This mechanism is supported by the fact that lower oxygen fractions at induction of anesthesia limit the development of intrapulmonary shunt.52 Mathematical modeling of gas exchange suggests that absorption atelectasis achieves a maximum as fast as 9 minutes if preoxygenation with 100% oxygen is performed over 3 minutes.53
An early study implicated the volatile anesthetic agents halothane and chloroform in the reduction of lung volume of excised lung dogs. In rabbit lungs, the combination of halothane with higher inspiratory oxygen concentrations increased the permeability of the alveolar–arterial barrier, possibly because of additional effects on the lung surfactant. Also in patients without pulmonary disease, the inhalation of volatile anesthetics reduced the surface tension of tracheobronchial secretions.54 Furthermore, inactivation of lung surfactant may occur as a result of large changes in alveolar surface area, which are associated with surfactant aggregate conversion.55
Chapter 37 discusses the effects of mechanical ventilation on the distribution of alveolar ventilation ( ) and perfusion (
) and perfusion ( ) in detail. Basically, mechanical ventilation is associated with
) in detail. Basically, mechanical ventilation is associated with 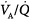 mismatching and increased intrapulmonary shunt, while diffusion limitation to O2 plays a marginal role. General anesthesia and surgery have the potential to aggravate the deterioration of gas exchange by further impairment of
mismatching and increased intrapulmonary shunt, while diffusion limitation to O2 plays a marginal role. General anesthesia and surgery have the potential to aggravate the deterioration of gas exchange by further impairment of 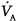 and diversion of perfusion from better ventilated lung zones.
and diversion of perfusion from better ventilated lung zones.
Induction of anesthesia with certain barbiturates (thiopental and thiamylal56), opioids (morphine57), and neuromuscular blocking agents (atracurium and D-tubocurarine58) induces histamine release by mast cells, possibly resulting in bronchoconstriction. Also, endotracheal intubation can elicit an increase of the smooth muscle tone of smaller peripheral airways.59 In contrast, volatile anesthetics, including halothane, isoflurane, sevoflurane, and desflurane, exert bronchodilatory effects.60
Patient positioning and the surgical procedure itself may affect the distributions of 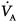 and
and  . During anesthesia in the supine position, a cephalad displacement of the diaphragm with reduction in FRC and impairment of
. During anesthesia in the supine position, a cephalad displacement of the diaphragm with reduction in FRC and impairment of 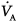 in juxtadiaphragmatic zones is commonly observed.61 Some authors, however, suggest that only the dorsal part of the diaphragm is shifted cranially, while the ventral part is displaced caudally.31 Such a displacement pattern has been attributed to anesthesia-induced changes in other chest wall structures, like the ribcage, where the diaphragm attaches. On the other hand, surgical procedures requiring the use of the Trendelenburg position and capnoperitoneum, which have more pronounced effects on the respiratory function than the supine position and open abdominal surgery,62 may lead to a displacement of the whole diaphragm. Interestingly, however, increased pressure in the lung base during the combination of the Trendelenburg position and laparoscopy also shifts
in juxtadiaphragmatic zones is commonly observed.61 Some authors, however, suggest that only the dorsal part of the diaphragm is shifted cranially, while the ventral part is displaced caudally.31 Such a displacement pattern has been attributed to anesthesia-induced changes in other chest wall structures, like the ribcage, where the diaphragm attaches. On the other hand, surgical procedures requiring the use of the Trendelenburg position and capnoperitoneum, which have more pronounced effects on the respiratory function than the supine position and open abdominal surgery,62 may lead to a displacement of the whole diaphragm. Interestingly, however, increased pressure in the lung base during the combination of the Trendelenburg position and laparoscopy also shifts  to lung apical zones, resulting in a preserved
to lung apical zones, resulting in a preserved 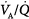 matching, despite deterioration in respiratory mechanics.63 During upper abdominal surgery, the use of retractors also importantly impair lung compliance64 and their effects on
matching, despite deterioration in respiratory mechanics.63 During upper abdominal surgery, the use of retractors also importantly impair lung compliance64 and their effects on 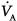 and
and  are probably the same as those of laparoscopic surgery.
are probably the same as those of laparoscopic surgery.
One-lung anesthesia has singular effects on 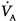 ,
,  , and
, and 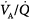 matching. Secondary to the collapse of the operated lung,
matching. Secondary to the collapse of the operated lung, 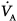 is limited to a single lung during such procedures. Gravity, however, slightly favors a shift of
is limited to a single lung during such procedures. Gravity, however, slightly favors a shift of  toward the dependent lung,65 resulting in a better-preserved gas exchange during one-lung anesthesia in the semilateral and lateral decubitus than in the supine position.66 Furthermore, hypoxic pulmonary vasoconstriction contributes to further redistribute the blood flow toward the ventilated lung. Theoretically, anesthesia has the potential to interfere with hypoxic pulmonary vasoconstriction. In dogs, volatile anesthetics cause pulmonary capillary dilation that may blunt the redistribution of blood flow and worsen oxygenation.67 The use of volatile anesthetics in patients, however, especially at the minimal alveolar concentration, has not been associated with worsening of oxygenation when compared to intravenous anesthetics.68–70 On the other hand, the use of epidural anesthesia in combination with intravenous anesthetics seems to result in better oxygenation by mechanisms not related to hypoxic pulmonary vasoconstriction, for instance, reduction of cardiac output.71
toward the dependent lung,65 resulting in a better-preserved gas exchange during one-lung anesthesia in the semilateral and lateral decubitus than in the supine position.66 Furthermore, hypoxic pulmonary vasoconstriction contributes to further redistribute the blood flow toward the ventilated lung. Theoretically, anesthesia has the potential to interfere with hypoxic pulmonary vasoconstriction. In dogs, volatile anesthetics cause pulmonary capillary dilation that may blunt the redistribution of blood flow and worsen oxygenation.67 The use of volatile anesthetics in patients, however, especially at the minimal alveolar concentration, has not been associated with worsening of oxygenation when compared to intravenous anesthetics.68–70 On the other hand, the use of epidural anesthesia in combination with intravenous anesthetics seems to result in better oxygenation by mechanisms not related to hypoxic pulmonary vasoconstriction, for instance, reduction of cardiac output.71
General anesthesia does not necessarily require controlled mechanical ventilation. In fact, for more than a century after its description in 1846, volatile anesthetics have been administered to spontaneously breathing patients.72 General anesthesia, however, may be associated with a reduction in FRC, tidal volume, changes in breathing pattern, and alterations in the mechanical properties of the respiratory system, resulting in impairment of oxygenation secondary to 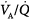 mismatching. Furthermore, the need to protect the airways from aspiration by means of endotracheal intubation and the use of neuromuscular blocking agents to facilitate surgery and endotracheal intubation itself led to the regular use of controlled mechanical ventilation to maintain an adequate gas exchange. Nevertheless, certain surgical procedures can be performed without neuromuscular blocking agents, and endotracheal intubation is not always necessary. In this context, facemasks, laryngeal masks, and other supraglottic devices have largely replaced endotracheal tubes. Accordingly, the interest of anesthesiologists in maintaining spontaneous breathing activity during general anesthesia has been increasing.
mismatching. Furthermore, the need to protect the airways from aspiration by means of endotracheal intubation and the use of neuromuscular blocking agents to facilitate surgery and endotracheal intubation itself led to the regular use of controlled mechanical ventilation to maintain an adequate gas exchange. Nevertheless, certain surgical procedures can be performed without neuromuscular blocking agents, and endotracheal intubation is not always necessary. In this context, facemasks, laryngeal masks, and other supraglottic devices have largely replaced endotracheal tubes. Accordingly, the interest of anesthesiologists in maintaining spontaneous breathing activity during general anesthesia has been increasing.
In view of these facts, anesthesiologists must be able to partially and fully assist the respiratory pump. For this purpose, several assisted spontaneous breathing and controlled mechanical ventilation modes that are present on ICU ventilators are required. Anesthesia ventilators, however, must be able also to deliver inhalation anesthetics in an environmental and economically friendly way, which is not feasible with semiopen systems. Table 24-1 lists the ventilator modes commonly used during anesthesia in the operating room. Obviously, not all anesthesia ventilators offer all of those modes and some of them are not available on anesthesia machines, as they are currently not appropriate for use in combination with volatile anesthetics—for example, modes based on high-frequency ventilation. However, developing technologies may overcome this limitation in the near future.
| Mode | Comment | Possible Indications in Anesthesia |
|---|---|---|
| CPAP (continuous positive airway pressure) | Increases mean airway pressure and allows spontaneous breathing | Induction of anesthesia to avoid major decrease of FRC; decrease inspiratory effort in the presence of an endotracheal tube; increase tidal volume during decreased inspiratory drive; before extubation to avoid loss of the FRC |
| PSV (pressure-support ventilation) | Increases the pressure at the airway above the expiratory pressure in response to an inspiratory effort; can be triggered either by flow or pressure | Maintenance of adequate ventilation in presence of reduced inspiratory drive, restrictive lung disease, partial neuromuscular blockade or neuromuscular disease; increased inspiratory effort in presence of a narrow airway device; reduction of atelectasis, improved gas exchange, decreased level of sedation;73 weaning from controlled ventilation |
| V- or PACV (volume or pressure assist-control ventilation) | Guarantees that the desired tidal volume or driving pressure is delivered upon an inspiratory effort | Allow patients with respiratory drive to control the respiratory rate and/or tidal volume |
| SIMV (synchronized intermittent mandatory ventilation; also SIMV-VC and SIMV-PC) | With SIMV, if an inspiratory effort is sensed either by pressure or flow during a sensitive time window, the ventilator applies a preset tidal volume (VC) or pressure (PC); a minimum mandatory ventilation can be set, which will guarantee acceptable gas exchange. SIMV has greater acceptance in the operating room than in the ICU | Maintenance of adequate ventilation in presence of inspiratory effort; emergence from anesthesia |
| VCV (volume-controlled ventilation) | VCV is time-cycled and volume-targeted; PCV is time-cycled and pressure-limited; corresponds to the default mode on most anesthesia ventilators | Tight control of tidal volume in the absence of inspiratory effort, allows better control of partial pressure of end-tidal carbon dioxide (PETCO2) |
| PCV (pressure-controlled ventilation) | PCV is time cycled and pressure-limited | Tight control of inspiratory peak pressure; possibly of value in combination with supraglottic airway devices to avoid gastric insufflation74 and upper airway surgery to avoid anastomose insufficiency. Also useful with uncuffed endotracheal tubes to compensate for leaks |
| VCV+AutoFlow | Used in the volume-controlled ventilation mode, autoflow automatically adjusts the inspiratory flow to achieve the desired tidal volume with lowest possible inspiratory pressure, resulting in an inspiratory decelerating flow pattern. It is also known as pressure-regulated volume-controlled ventilation (PRVCV) and is similar to pressure control with volume guarantee (PC-VG) | In combination with a supraglottic airway or surgery of the upper airways to reduce peak pressures and avoid gastric insufflation and airway anastomose insufficiency |
| APRV (airway pressure release ventilation) | Time-cycled ventilation with a higher continuous airway pressure that is maintained for longer periods of time (usually >2 s) and periodically released until positive end-expiratory pressure (PEEP) for short periods of time (usually <1 s, typically 0.5 s), resulting in an inverse inspiratory-to-expiratory timing (I:E) ratio. Free (nonassisted) spontaneous breathing activity is possible at any time at the higher airway pressure | Mainly for patients with acute lung injury and/or acute respiratory distress syndrome to increase mean airway pressure and functional residual capacity; may be helpful to limit the pressure in the airways at comparatively higher levels, while allowing spontaneous breathing (e.g., in combination with supraglottic airway devices) and promoting CO2 washout |
| BIPAP (biphasic [intermittent] airway pressure ventilation) | Works similarly to APRV, changing the airway pressure between two levels but allowing more time for expiration than inspiration. BIPAP can be used with time windows that sense inspiratory and expiratory efforts and in combination with pressure-supported breaths at the lower airway pressure level. Also known as BiPAP (bi-level positive airway pressure). A variant called Bi-Vent also provides pressure support for spontaneous breaths at the higher airway pressure level | Similar indications as for PCV and PSV; smoother transition from controlled to assisted spontaneous breathing; maintenance of adequate ventilation in presence of reduced inspiratory drive, restrictive lung disease, partial neuromuscular blockade, or neuromuscular disease |
| HFJV (high-frequency jet ventilation) | Usually with respiratory frequencies of 100 to 150 breaths/min and pressure of 1.5 to 2.5 bars; useful with infraglottic, supraglottic, transtracheal, and transluminal airway devices | Surgery and diagnostic procedures of the upper respiratory tract, ventilation of the dependent lung during one-lung anesthesia, emergency transtracheal ventilation75 |
| HFOV (high-frequency oscillatory ventilation) | Usually with respiratory frequencies of 600 to 900 breaths/min and increased mean airway pressure; active instead of passive expiration; requires higher bias gas-flow rates. The resulting tidal volume is relatively low and, in case of CO2 retention, can be increased by reducing the respiratory frequency | Ventilation of the dependent lung during one-lung anesthesia, CT-guided percutaneous procedures of the kidneys to reduce movement artifacts, thoracic and abdominal neonatal surgery where an increase of mean airway pressure is indicated (e.g., congenital diaphragmatic hernia, esophageal atresia, correction of abdominal wall defects)76 |
The main goals of mechanical ventilation during general anesthesia are to oxygenate arterial blood and secure adequate CO2 elimination.77 To achieve those aims, tidal volumes as high as 12 to 15 mL/kg of predicted body weight for two-lung ventilation, and 8 to 10 mL/kg for one-lung ventilation have been advocated and represent common practice. It was recognized early that the use of high tidal volumes helped in avoiding intraoperative lung atelectasis40 and decreased FRC. Accordingly, the use of positive end-expiratory pressure (PEEP) has been primarily linked to its potential for increasing FRC and avoiding airway closure, even though PEEP does not necessarily improve oxygenation during general anesthesia.78,79 Because of the potential harmful effects of PEEP, however, especially on hemodynamics, its use during anesthesia does not represent the standard of care.
There is unequivocal evidence that mechanical ventilation can worsen injury in previously damaged lungs. On one hand, high tidal volumes may promote overdistension of the alveoli (volutrauma). On the other hand, the lack of PEEP can lead to destabilization of lung units at end-expiration, resulting in repeated collapse and opening during tidal ventilation (atelectrauma). The mechanical stress caused by volutrauma and atelectrauma can be translated into the activation of the inflammatory cascade, culminating in the release of proinflammatory mediators by cells, a phenomenon known as mechanotransduction and that represents the basis of the so-called biotrauma. The exact mechanisms by which mechanical stress is sensed and then transduced into inflammation are not fully elucidated, but activation of stretch-sensitive channels, partial cell membrane disruption, and conformational changes in membrane-associated molecules and cytoskeletal structure have been implicated in the gene expression and production of cytokines.80 Following, and most probably also simultaneously with the proinflammatory response, the remodeling process can be activated, with upregulation of gene expression and synthesis of collagen types I and III fibers by fibroblasts.81–83 Furthermore, the mediators involved in repair and remodeling can also trigger the expression of metaloproteinases,84,85 enzymes that promote the degradation of components of macromolecules of the extracellular matrix, namely proteoglycans and glycosaminoglycans, as well as fibrous (collagen and elastin) and structural or adhesive proteins (fibronectin and laminin).86 In addition, the mechanical stress generated by high tidal volumes also elicits changes in the expression of some proteoglycan components of the extracellular matrix, possibly changing its mechanical properties.87 In fact, fragmentation of pulmonary glycosaminoglycans has been detected with tidal volumes as low as 8 mL/kg in healthy rats, resulting in decreased lung compliance and edema.88
Although mechanical stress per se is able to activate the proinflammatory and remodeling responses in noninjured lungs, tidal volumes as high as 22 mL/kg do not exceed the threshold of stress and/or strain necessary to cause lung weight gain, systemic inflammation, and multiple organ dysfunction in healthy pigs.89 In line with these findings, nonprotective mechanical ventilation with high tidal volume and zero PEEP did not increase the release of inflammation mediators in surgical patients without lung disease.90–92 Such reports support the hypothesis that ventilator-associated lung injury occurs only if the lungs are predisposed to injury by a first hit, that is, an inflammatory process already present in the lungs (the two-hit hypothesis). In this context, inappropriate mechanical ventilation would represent the second hit that elicits lung injury. The two-hit hypothesis, however, has been challenged. In patients undergoing one-lung anesthesia, protective mechanical ventilation with low tidal volume and PEEP decreased the proinflammatory systemic response after esophagectomy.93 Also during two-lung anesthesia, the use of low tidal volume and PEEP attenuated the levels of interleukin (IL)-8 and myeloperoxidase in bronchoalveolar lavage fluid in patients without preexisting lung injury.94 Furthermore, in patients admitted to the ICU, large tidal volumes represent a major risk factor for the development of acute lung injury (ALI).95,96 Possibly, cytokines released by surgical trauma and surgery-related conditions, for instance, ischemia or reperfusion of different organs, as well as subclinical lung inflammation, could function as a first hit, and thus explain those discrepancies.
Whether protective mechanical ventilation strategies during anesthesia in patients without lung injury may avoid the onset of ALI, the acute respiratory distress syndrome, or other forms of pulmonary complications remains to be determined.
Approximately 234 million major surgical procedures are performed every year worldwide, with 2.6 million representing high-risk procedures.97 About half of the patients undergoing high-risk interventions develop complications and 315,000 die during their hospital stay. Postoperative pulmonary complications are as common as cardiac complications following noncardiac surgery98 and occur in approximately 5% of all patients undergoing surgery and anesthesia.99 This number, however, increases importantly in the presence of certain risk factors, including older age, low preoperative oxygen saturation, respiratory infection in the last 30 days preceding surgery, anemia (hemoglobin level <10 g/dL), surgery of the upper abdominal or thorax, surgery lasting longer than 2 hours, and emergency procedures.99
The incidence of postoperative pulmonary complications also vary according to their definition,98,100–102 and the following entities and/or findings have been classified as a postoperative pulmonary complication: respiratory failure from pulmonary or cardiac origin; pneumonia and respiratory infection; pleural effusion and atelectasis; pneumothorax; bronchospasm; and need for noninvasive respiratory support or reintubation. Once a postoperative pulmonary complication occurs, the average hospital stay is prolonged and the risk of in-hospital death increases.99,103
Different strategies have been proposed to reduce the incidence of postoperative pulmonary complications, including postoperative lung-expansion maneuvers, preoperative intensive inspiratory muscle training, selective rather than routine use of nasogastric tubes, use of short-acting rather than long-acting neuromuscular blockade, and laparoscopic instead of open bariatric surgery.98,104,105 Despite the compelling rationale for the use of PEEP (combined or not with lung-recruitment maneuvers) to prevent formation of atelectasis in the intraoperative period, an increase of FRC did not improve outcome following surgery in a nonselected population.106 The outcome might be different if patients with higher risk for postoperative pulmonary complications were selected. For this purpose, different propensity scores are available, as shown in Table 24-2. The major weakness common to all of those prediction models is that events and interventions in the intraoperative period, for example, bleeding and hemodynamic instability, are not taken into account and may impair their accuracy. Accordingly, the impact of the intraoperative ventilation strategy is not taken into account. In view of these facts, such scores require validation in independent trials.
| Ref | Type of PPC | Predictors of PPCs | Type and Number of Patients/Setting |
|---|---|---|---|
| Christenson 1996107 | ARDS (1%) |
| Cardiac surgery with cardiopulmonary bypass (n = 3848) |
| Kutlu 2000108 |
|
| Pulmonary resection (n = 1139) |
| Tandon 2001109 | ARDS (14.5%), pleural effusion (15%), prolonged pneumothoraces (4.1%), hydropneumothoraces (1.8%), empyema (2.4%), chylothorax (2.4%), pneumonia (17.8%), pulmonary embolism (1.8%), laryngeal edema (0.5%) |
| Elective esophagectomy (n = 168) |
| Milot 2001110 |
|
| Cardiac surgery with CPB (n = 3278) |
| Licker 2003111 |
|
| Thoracic surgery for non–small-cell lung carcinoma (n = 879) |
| Fernandez-Perez 2006112 | Total PPC (18%): acute lung injury (50%), cardiogenic pulmonary edema (17%), pneumonia (23%), bronchopleural fistula (7%), pulmonary thromboembolism (3%) |
| Pneumonectomy (n = 170) |
| Alam 2007113 | Total PPC (5.3%): ALI or ARDS (3.1%), pneumonitis (2.2%) |
| Thoracic surgery. lung resection (n = 1428) |
| Johnson 2007114 | Total PPC (3%): pneumonia (35.%), systemic sepsis (23.%), and cardiac arrest (13.3%) |
| Major general or vascular procedures (n = 180,359) |
| Canet 201099 | Total PPC (5%): respiratory infection (1.6%), respiratory failure (2.6%), bronchospasm (1.8%), atelectasis (1.4%), pleural effusion (1.7%), aspiration pneumonitis (0.4%) |
| Surgical procedures (general, neuraxial, or regional anesthesia) (n = 2464) |
| Sen 2010115 |
|
| Pulmonary resection (n = 143) |
| Ferguson 2011116 | PPC (38%) |
| Esophagectomy (n = 516) |
| Kor 2011117 | ALI (2.6%) |
| High-risk surgery (n = 4366) |
Anesthesia Ventilators
General anesthesia was first delivered using an open-inhaler system by John Snow as early as 1846. Because anesthesia itself often resulted in depression of the respiratory motor output, ventilation had to be assisted by bag ventilation. Thus, besides vaporizers, anesthetic circuits, and scavenging systems, mechanical ventilators became a fundamental part of the anesthesia machine as a means to automate ventilation. Mechanical ventilators, however, represent nowadays more than simple bag substitutes. Rather, they play a central role in the anesthesia machine and must match different requirements than their counterparts in the ICU, justifying the use of the term anesthesia ventilator.
Most commonly, the anesthesia ventilator is used in combination with the so-called circle system, which is depicted in Figure 24-2. That system includes an adjustable airway-pressure limiting valve that can be set by the anesthesiologist to limit airway pressure during bag ventilation, a bag ventilation valve itself with a respective on/off valve, an entrance for a fresh mixture of respiratory and anesthetic gases, a scavenging system, an absorber for CO2, and the anesthesia ventilator. During the expiratory phase, the anesthesia ventilator is filled with a gas mixture coming from the patient, which is pressed again into the anesthesia circuit during inspiration. In other words, such system permits exhaled air to be rebreathed by the patient, thus reducing the consumption of anesthetic gas agents. In general terms, the anesthesia ventilator is embedded in a semiclosed system in contrast to ICU ventilators, which represent open or semiopen systems.
Figure 24-2
Schematic of the circle system used in most anesthesia machines. Fresh gas consisting of a mixture of oxygen, nitrogen, and anesthetic gases flows into the circle system between the CO2 canister, which contains a CO2 absorber salt, and the inhalation check valve. Gas exhaled by the patient passes an exhalation check valve and refills the anesthesia ventilator and/or reservoir bag. Because the flow rate of fresh gas can be as low as the gas consumption rate, the circle system can recirculate most of the exhaled gas. The anesthesiologist can ventilate the patient manually by bag or using an anesthesia ventilator upon switching of a selector valve. During bag ventilation, the adjustable pressure-limiting (APL) valve is set to limit the maximal pressure in the circuit, while, during function of the anesthesia ventilator, the maximal pressure is usually fixed at 70 cm H2O by an internal pressure-relief valve. (Adapted from Andrews JJ. Inhaled anesthetic delivery systems. In: Miller RD, ed. Anesthesia. New York: Churchill Livingstone; 1994.)
Chapter 2 presents a comprehensive scheme for classification of mechanical ventilators, which also applies for devices embedded in anesthesia machines. The classification of anesthesia ventilators, however, according to: power source, driving mechanism, cycling mechanism, and design, that is, bellows, piston, or turbine, is useful for the anesthesiologist, because it allows inference of expected performance and safety aspects of the anesthesia machine.
Some anesthesia ventilators are powered by compressed gas, but most modern ventilators are operated either by electricity alone or a combination of electricity and compressed gas. When compressed gas is used, the ventilator can function even in the absence of electricity.
Several anesthesia machines have high-pressure and low-pressure circuits, that is, are a double-circuit. In the low-pressure circuit, the mixture consisting of anesthetic and respiratory gases are contained in a bag or bellows. Gas at higher pressure, usually oxygen (sometimes mixed with air), compresses the bellows to move the anesthetic gas mixture into the lungs.
The cycling mechanism describes how the ventilator initiates inspiration in the control mode. Most contemporary anesthesia ventilators are time cycled and use solid-state electronics for timing. Older devices may be based on pneumatic and fluidic timers.
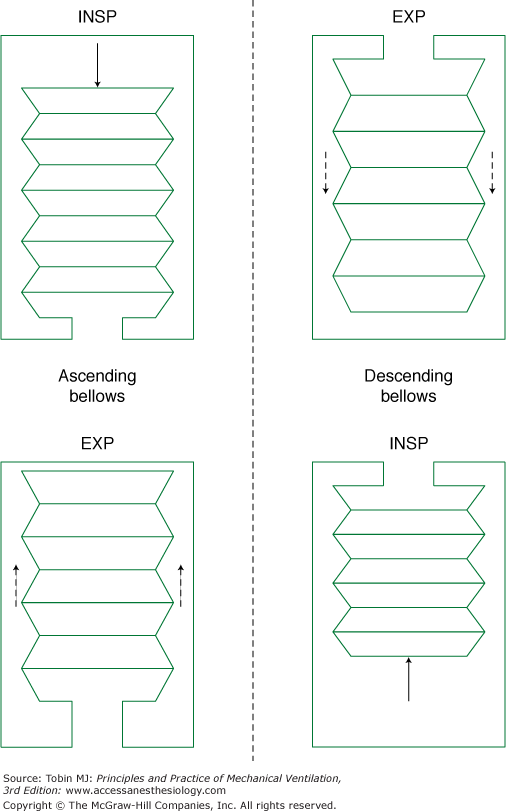
The first anesthesia ventilators were based on the bellows design. Bellows ventilators more or less simply automate bag ventilation, and can be still found in modern anesthesia machines. Bellows are located in a chamber where a driving gas flows in to generate an external pressure that squeezes out their contents. The driving gas mixture, which usually consists of oxygen (sometimes mixed with air), is completely separated from the anesthetic gas mixture delivered to the patient.
Bellows are classified according to their motion during expiration, as shown in Figure 24-3. Accordingly, ascending (standing) bellows move upwards, while descending (hanging) bellows move downwards in the expiratory phase. Alternatively, bellows may be displaced horizontally, minimizing the effect of gravity on bellows movement. In addition, horizontal bellows that have a large surface area offer reduced mechanical resistance to movement.
Given that the weight of a descending bellows promotes its expansion, it may continuously inflate and deflate even in the absence of adequate patient ventilation, for instance during patient disconnection from the anesthesia circuit. Because of this serious safety concern, most bellows ventilators in anesthesia machines are of the ascending type. During inspiration, a driving gas squeezes the bellows downward, pushing its content into the anesthesia circuit. Simultaneously, the driving gas closes a relief valve, avoiding escape of the anesthetic gas mixture into the scavenging gas system. During expiration, the pressure around the bellows is reduced to zero, allowing escape of the driving gas and upward movement of the bellows, which is mainly driven by the pressure in the patient’s airways. Because the ventilator relief valve opens first at 2 to 3 cm H2O during expiration, the bellows are filled first before any gas is scavenged, and a minimal PEEP of 2 to 3 cm H2O results. Another problem associated with the bellows anesthesia ventilator is that fresh gas flow is permanently added to the anesthesia circuit during the whole-breath cycle. Accordingly, the effectively delivered tidal volume may differ from the anesthesia ventilator settings depending on inspiration time. Furthermore, the compliance of the breathing circuit may also result in differences between desired and delivered tidal volume.
The problem of fresh gas flow-dependence on tidal volume is not exclusive to bellows anesthesia ventilators and can be fixed by decoupling systems that are present in some anesthesia machines. For instance, the fresh gas can be decoupled from the anesthesia circuit by a valve that closes during inspiration, diverting the fresh gas to the bag reservoir during inspiration. Furthermore, flow and volume sensors placed near the patient–airway interface may be used to compensate for differences between desired and effectively delivered tidal volume (VT). Such differences may result not only from inappropriate fresh gas flow rates, but also from expansion of the breathing circuit during inspiration. Those sensors, however, are prone to accumulation of moisture, which may impair their accuracy, jeopardizing the performance of the ventilator.
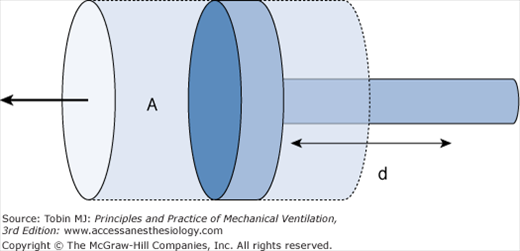
The use of mechanical ventilators based on piston (Fig. 24-4) instead of bellows design in anesthesia machines is increasing. Anesthesia piston ventilators are able to deal more efficiently with volume compensation secondary to compliance of the breathing circuit because they depend not on measurement of airflow or volume but rather pressure, which is less sensitive to the accumulation of moisture or secretions. Furthermore, control of the piston’s displacement allows more accurate volume delivery, especially in situations where low tidal volumes are required, for example, in neonatal anesthesia. In addition, the piston ventilator allows a more rapid increase in flow than a bellows ventilator, which is particularly important for pressure-controlled ventilation (PCV).
Because of the ease and accuracy with which the piston can be controlled, advanced ventilation modes can be implemented through software enhancements to the piston ventilator. The basic piston design has proven itself to be a versatile platform for anesthesia ventilator design, which permits transferring differentiated mechanical ventilation modes from the ICU to the operating room.
An innovative anesthesia ventilator design is the electrically driven and controlled compressor turbine. The turbine has a two particularities that make it attractive for anesthesia ventilators:118 (a) it builds up the breathing pressure and delivers the corresponding flow to the patient during inspiratory time; and (b) it delivers a circuit flow that is required to mix the gas within the breathing system independently of patient inspiratory effort. During the inspiratory phase of controlled ventilation, the turbine transports the breathing gas from the breathing bag reservoir to the patient. During expiration, the gas returns to the bag and is additionally circulated and mixed.118 This anesthesia ventilator design is particularly important for closed-systems, which allow minimal consumption of anesthetic gases, that is, quantitative anesthesia. Because a turbine-based ventilator performs as well as the best compressed-gas ICU ventilators,119 it offers a useful platform for implementation of advanced mechanical ventilation modes in anesthesia.
Table 24-3 depicts the basic characteristics of some available anesthesia ventilators. For a complete and comparative description of those devices we recommend the reader to assess the homepage of the nonprofit research agency ECRI Institute at www.ecri.org.
| Fabricant | Dräger Medical | Dräger Medical | Dräger Medical | Dräger Medical |
|---|---|---|---|---|
| Model | Zeus IE | Primus IE | Fabius GS Premium, Fabius MRI | Cicero EM, Cicero B |
| Ventilator design | Turbine | Piston | Piston | Piston |
| Bellows size | — | — | — | — |
| Ventilation modes* | Manual/spontaneous, CPAP, VCV, Autoflow, PCV, SIMV-PS, PSV | Manual/spontaneous, CPAP, VCV, Autoflow, PCV, SIMV-PS, PSV | Manual/spontaneous, CPAP, VCV, PCV, SIMV, PSV | Manual/spontaneous, CPAP (APL valve), VCV, PCV, SIMV |
| Tidal volume (mL) | 20 to 1500 | 20 to 1400 | 20 to 1400 | 20 to 1400 |
| Minute volume (L/min) | Up to 40 | Up to 50 | Up to 50 | — |
| Respiratory rate (breaths/min) | Up to 80 | 3–100 | 4–60 | 6 to 80 (VCV, PCV) 3 to 80 (SIMV) |
| Inspiratory flow (L/min) | 180 maximum | 150 maximum | 10 to 75 in PCV/10 to 85 in PS and SIMV | 5 to 75 |
| I:E ratio | 4:1 to 1:4 | 5:1 to 1:99 | 4:1 to 1:4 | 1:3 to 2:1 |
| Inspiratory pause (% of TI) | 20 to 50 | 0 to 60 | 0 to 50 | 0 to 60 |
| Pressure limit (cm H2O) | Up to 70 | Up to 70 | Up to 70 | Up to 70 |
| PEEP (cm H2O) | 0 to 35 | 0 to 20 (Pmax-10 hPa in volume mode; Pmax-6 hPa in pressure mode) | 0 to 20 | 0.2 to 15 |
| Control of inspiratory tidal volume | Fresh gas decoupling and compliance compensation | Fresh gas decoupling and compliance compensation | Fresh gas decoupling and compliance compensation | Fresh gas decoupling and compliance compensation |
| Fabricant | GE Healthcare | MEDEC | Spacelabs Healthcare | |
| Model | Aisys Carestation, Avance | Neptune, Saturn Evo Color, Saturn Evo Standard | Blease Focus, Blease Serius | |
| Ventilator design | Bellows (ascending) | Bellows (horizontal bag-in-bottle) | Bellows (ascending bag-in-bottle) | |
| Bellows size | 1500 mL | 1 for neonate to adult | Pediatric/adult | |
| Ventilation modes* | Manual/spontaneous, CPAP, VCV, PCV, PCV-VG, SIMV-VC, SIMV-PC, PSVPro, end-tidal control (not in the Avance) | Manual/spontaneous, VCV, PCV, SIMV, PSV | Manual/spontaneous, VCV, (Precision) PCV, SIMV-PS | |
| Tidal volume (mL) | 20 to 1500 | 10 to 1600 | 20 to 1500 | |
| Minute volume (L/min) | 0.08 to 120 | Not specified | 0.3 to 25 | |
| Respiratory rate (breaths/min) | 4 to 100 | 4 to 80 | 2 to 99 | |
| Inspiratory flow (L/min) | 1 to 120 | Automatic, decelerating flow pattern | 0 to 100, variable | |
| I:E ratio | 2:1 to 1:8 | 4:1, 3:1, 2:1, 1:1, 1:1.5, 1:2, 1:3, 1:4, 1:5, 1:6 | 2:1 to 1:5 | |
| Inspiratory pause (% of TI) | 0 to 60 | 0 to 50 | 0 to 50 | |
| Pressure limit (cm H2O) | 12 to 100 | 7 to 99 | 10 to 70, adult 10 to 50, pediatric | |
| PEEP (cm H2O) | 4 to 30 | 0 to 20 | 3 to 20 | |
| Control of inspiratory tidal volume | Fresh gas decoupling and compliance compensation | Compliance compensation | Fresh gas decoupling and compliance compensation, tidal volume increased by 10% at every tenth breath (sigh function) |
Mechanical Ventilation Strategies during General Anesthesia
Mechanical ventilation strategies can influence the development of ventilator-induced lung injury and affect clinical outcome following surgery. Despite a compelling rationale for the use of lower VT, PEEP, and recruitment maneuvers, the role of protective mechanical ventilation during general anesthesia has not been established. Nevertheless, several clinical trials have addressed the impact of mechanical ventilation on pulmonary function, lung injury, and clinical outcome (Table 24-4).
| Control Group | Protective Group | Inflammation Markers | Intraoperative Outcomes | Postoperative Outcomes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref | Type and Number Patients/Setting | PEEP | VTmL/kgIBW | PEEP | VTmL/kgIBW | TNFα | IL-1 | IL-2 | IL-6 | IL-8 | IL-10 | compl | oxy | oxy | atelect | PPC | Length of Stay |
| Lee 1990120 | Postoperative pts (not neurosurgical) (n = 103)/ICU | — | 12 | — | 6 | NA | |||||||||||