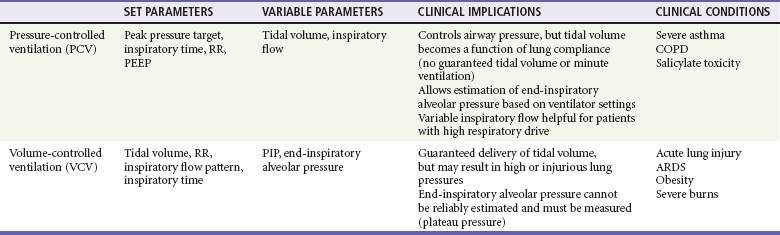
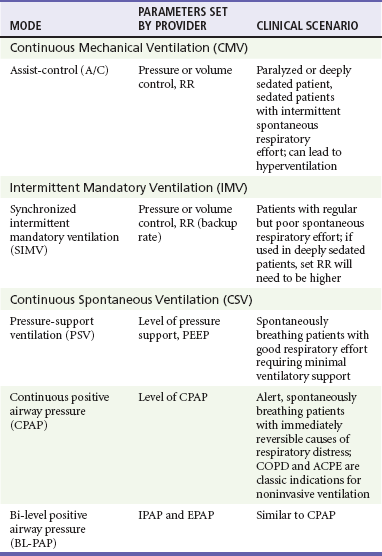
Chapter 2 The decision to intubate is discussed in Chapter 1 and in various other places throughout this textbook in the context of individual conditions. This chapter describes the modalities and techniques of noninvasive and invasive mechanical ventilation. Physiology of Positive Pressure Breathing Transition from negative pressure breathing to positive pressure breathing affects cardiovascular and pulmonary physiology and can have significant clinical consequences. Pressure changes in the thoracic cavity directly affect pressures in the chambers of the heart. During spontaneous inspiration, venous return and preload are augmented, cardiac output is increased, and there is an increased pressure gradient between the left ventricle and the aorta. With the initiation of positive-pressure ventilation (PPV), venous return is diminished, cardiac output falls, and there is a decreased pressure gradient between the left ventricle and the aorta.1 Relative hypotension can occur after ventilatory support has been initiated, and this may be exaggerated in patients with clinical hypovolemia or vasodilatory states.2 In VCV a breath is defined by delivery of a set tidal volume to the lungs. Inspiratory flow is fixed, and inhalation ends when a preset tidal volume has been delivered; peak inspiratory pressures (PIPs) and end-inspiratory alveolar pressures vary based on lung compliance and set tidal volume. The main benefit to VCV is the ability to control tidal volume and minute ventilation, but it may be problematic by causing high peak pressures when the compliance of the respiratory system is poor. Clinically, poor respiratory system compliance occurs in conditions that increase lung or chest wall stiffness. Such conditions include pulmonary edema, acute lung injury (ALI) or acute respiratory distress syndrome (ARDS), pneumothorax, and obesity.3 When a physician is choosing between pressure-cycled ventilation versus volume-cycled ventilation, it is important to consider the underlying reason for mechanical ventilation. Volume-cycled ventilation should be used when strict control of tidal volume is mandated. Specifically, this includes patients with known ALI or ARDS, in whom low–tidal volume strategies have been proven to reduce mortality.4 In addition, patients with decreased chest wall compliance should be placed on VCV to ensure that adequate tidal volume is delivered. This includes patients with morbid obesity or severe chest wall burns. Conversely, in conditions in which strict control of airway pressure is desired, pressure-cycled ventilation should be used. As detailed earlier, this includes patients with asthma or COPD. In addition, because inspiratory flow is not limited in pressure-cycled ventilation, this strategy may be preferred to volume-cycled ventilation in patients with a high respiratory drive, such as patients with salicylate overdose. For patients who do not require strict control of either pressure or volume, similar ventilation mechanics can generally be achieved with either pressure-cycled or volume-cycled ventilation (Table 2-1). Ventilator mode refers specifically to the amount of respiratory support provided by the ventilator but more commonly represents a combination of the type of breath to be given and the way the breath is to be initiated. The most common ventilator modes can be categorized on the basis of how often the ventilator will initiate a breath for the patient and can be divided broadly into continuous mechanical ventilation (CMV), intermittent mechanical ventilation (IMV), and continuous spontaneous ventilation (CSV).5 CMV and IMV can be delivered via pressure-control or volume-control methods. In CSV, no mandatory breaths are delivered to a patient; the size of the breath is determined by the effort of the patient and can be augmented with applied pressure to the airway. These methods are compared in Table 2-2. Other, more complex modes of ventilation include proportional-assist ventilation (PAV) and airway pressure release ventilation (APRV), though these generally are not used in the emergency department (ED). CMV is intended to provide full ventilatory support for patients with little or no spontaneous respiratory activity. CMV, also referred to as assist-control (A/C) ventilation, provides a preset number of breaths per minute. In addition to preset breaths, A/C will also deliver a breath in response to patient effort. In this mode, patients can trigger a breath at any rate but will always receive at least the preset number of breaths. Notably, when a patient initiates a breath, he or she receives the full breath as set on the ventilator. For the promotion of ventilator synchrony, a spontaneous patient-initiated breath will take priority over a preset breath, meaning that if the ventilator is set to deliver 12 breaths/min, a breath is provided every 5 seconds in the absence of spontaneous inspiratory effort. When the patient makes a spontaneous effort, the ventilator provides an additional breath and the timer resets for another 5 seconds. A/C ventilation is the most useful initial mode of mechanical ventilation in ED patients, as many patients are initially paralyzed and sedated and do not interact with the ventilator. One of the biggest challenges with A/C ventilation, however, is that patient-initiated breaths are not proportional to patient effort; when inspiratory effort is detected, a full-sized breath is delivered. Clinically, this requires adequate sedation of patients when ventilated in A/C mode to prevent spontaneous respiratory efforts that will result in hyperventilation, air trapping, hypotension, and poor ventilator synchrony.6 Regardless of the ventilatory mode chosen, PEEP is often used during invasive mechanical ventilation. PEEP refers to the maintenance of positive airway pressure after the completion of passive exhalation. During acute respiratory failure, lung volumes are typically decreased; application of PEEP increases functional residual capacity (FRC), improves oxygenation, and decreases intrapulmonary shunt. Use of PEEP also reduces portions of nonaerated lung that may contribute to the development of ventilator-induced lung injury7 Notably, PEEP increases both intrapulmonary and intrathoracic pressure and may affect pulmonary and cardiovascular physiology. Potential adverse effects of PEEP include decreased cardiac output, lung overdistention, and pneumothorax. General Approach: Noninvasive versus Invasive Ventilation The decision to intubate carries significant implications for patients, including potentially life-threatening complications related to airway management and subsequent complications related to intensive care unit (ICU) care.8 NPPV is an appealing option for patients requiring ventilatory assistance with potentially reversible conditions when tracheal intubation is not immediately necessary, or as a therapeutic adjunct for patients with “do-not-intubate” directives.9,10 In appropriately selected patients, NPPV obviates intubation in greater than 50% of cases and improves survival.8,11 Need for emergency intubation is a contraindication to NPPV, except as a means to improve preoxygenation in preparation for intubation. Other relative contraindications include decreased level of consciousness, lack of respiratory drive, increased secretions, hemodynamic instability, and conditions, such as facial trauma, that would prevent an adequate mask seal.12,13 If NPPV is initiated, patients should be reassessed frequently for progress of therapy, tolerance of the mode of support, and any signs of clinical deterioration that indicate a need for intubation. Patients most likely to respond to NPPV in the ED are those with more readily reversible causes of their distress, such as COPD exacerbation or cardiogenic pulmonary edema in which fatigue is a significant factor. Robust evidence suggests benefit of NPPV for both conditions. In patients with acute COPD exacerbations, NPPV decreases the need for subsequent intubation, hospital length of stay, and mortality when compared with standard therapy. Notably, though helpful in most patients with COPD exacerbation, NPPV has been shown to have the largest benefit for patients with hypercapnic acidosis and pH below 7.3.14–17 Treatment failure, defined as subsequent need for intubation, is predicted by a Glasgow Coma Scale score of less than 11, a sustained arterial pH less than 7.25, and tachypnea greater than 30 breaths/min.18 In patients with acute cardiogenic pulmonary edema (ACPE), NPPV reduces the work of breathing while simultaneously improving cardiac output; application of NPPV causes elevations in intrathoracic pressure that decrease both left ventricular (LV) ejection pressure and LV transmural pressure. This results in afterload reduction. In addition, decreases in RV preload may improve LV compliance via ventricular interdependence.1–3,19,20 Compared with standard therapy, multiple studies and several meta-analyses have confirmed decreased need for intubation as well as decreased mortality for patients with ACPE treated with NPPV. Benefits were independent of whether patients received CPAP or BL-PAP, and despite suggestions from early clinical data, no increased rate of acute myocardial infarction occurred in patients receiving any form of NPPV.21–24 Though either modality can be used, a recent ED-based study suggested faster clinical improvement with BL-PAP.25 Specific predictors of failure of NPPV in congestive heart failure (CHF) have not been systematically examined. Use of NPPV in other patients with respiratory compromise, including asthma and pneumonia, is not well studied, though limited preliminary data suggest that NPPV may be beneficial for patients with acute asthma exacerbations.26–28 Studies have failed to establish a role for NPPV in pneumonia. Although no data suggest harm from NPPV, the presence of pneumonia has been shown to be an independent risk factor for failure of noninvasive ventilation.29–32 Initial settings for noninvasive ventilation should be determined by the amount of ventilatory assistance required by the patient, as well as patient comfort and cooperation with the therapy. The first consideration in the use of NPPV is whether to provide support in the form of CPAP or BL-PAP. This was discussed earlier, and there is no clear benefit of one over the other. Support will be given by a full-face (oronasal) mask or nasal mask; this choice is determined by patient comfort, feelings of claustrophobia, and the need for the patient to effectively cough or speak. Inspiratory support (IPAP) can be initiated at 10 cm H2O, and expiratory support (EPAP) can be initiated at 5 cm H2O. Subsequent titration of these parameters is based on the patient’s clinical response, particularly pressure tolerance, respiratory rate, and oxyhemoglobin saturation. Though blood gas analysis is confirmatory, improvement in clinical condition can be observed by decrease in work of breathing, good patient-ventilator synchrony, and patient report. If required, EPAP and IPAP can be adjusted by 1 to 2 cm H2O at a time based on clinical response.12 If work of breathing is unchanged, increases in IPAP and EPAP reduce hypercarbia by increasing tidal volume and minute ventilation while increasing oxygenation by combating atelectasis and promoting alveolar recruitment. IPAP greater than 20 cm H2O can be uncomfortable and can cause gastric insufflation and should be avoided.12,33 For the intubated patient, initial ventilator settings should facilitate ventilation that improves gas exchange, promotes ventilatory synchrony, and minimizes the potential for complications. For an apneic or paralyzed patient, full ventilatory support is required; for this reason, A/C is the recommended mode of initial ventilation. Specific required settings depend on whether the patient is receiving PCV or VCV, but the principles underlying selection of settings are similar. Reasonable initial ventilator settings should deliver a tidal volume of 6 to 8 mL/kg of estimated ideal body weight (IBW) at rate of 12 to 14 breaths/min. If VCV is used, tidal volume can be set directly, and if PCV is used, tidal volume is determined by adjusting the targeted pressure to be delivered; initial pressure targets should not exceed 30 cm H2O. Initial FIO2 should be set at 1.0 but generally can be adjusted down quickly to maintain an oxygen saturation of 90% or greater. PEEP is routinely given and is set initially at 5 cm H2O.6 Settings for specific clinical conditions, such as status asthmaticus, are discussed later.
Mechanical Ventilation and Noninvasive Ventilatory Support
Perspective
Principles of Mechanical Ventilation
Invasive Mechanical Ventilation
Positive End-Expiratory Pressure
Management
Approach to Initial Ventilator Settings

Full access? Get Clinical Tree