48 Mechanical Ventilation
Positive-pressure mechanical ventilatory support provides pressure and flow to the airways to effect oxygen (O2) and carbon dioxide (CO2) transport between the environment and the pulmonary capillary bed. The goal is to maintain appropriate levels of partial pressure of O2 and CO2 in arterial blood while unloading the ventilatory muscles. Conceptually, mechanical ventilatory support can be either total or partial. With total support, the mechanical device is designed to provide virtually all the work of breathing. Although patient effort may be present and may trigger ventilator breaths or even provide a small number of spontaneous breaths, total support should provide virtually all needed minute ventilation, with minimal patient contributions. In contrast, with partial support, the mechanical device is designed to only partially unload ventilatory muscles, requiring the patient to provide the remainder of the work of breathing. In general, total support is used in acute respiratory failure when the patient’s muscles are overloaded or fatigued or when gas exchange is very unstable or unreliable. Partial support is generally used in less severe forms of respiratory failure (especially during the recovery or weaning phase). Partial support issues are discussed in Chapters 49 and 50. This chapter focuses on positive-pressure ventilation designed to provide total support.
 Device Design Features for Total Ventilatory Support
Device Design Features for Total Ventilatory Support
Positive-Pressure Breath Controller
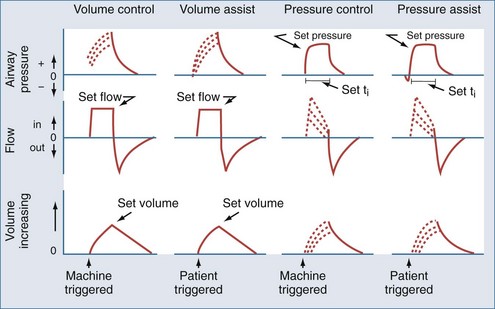
Most modern ventilators use piston-bellows systems or high-pressure gas sources to drive gas flow.1,2 Tidal breaths are generated by this gas flow and can be classified in terms of what initiates the breath (trigger variable), what controls gas delivery during the breath (target or limit variable), and what terminates the breath (cycle variable).3 During total support, breaths can be initiated (triggered) by patient effort (assisted breaths) or by the machine timer (controlled breaths). Target or limit variables are generally either a set flow or a set inspiratory pressure. With flow targeting, the ventilator adjusts pressure to maintain a clinician-determined flow pattern; with pressure targeting, the ventilator adjusts flow to maintain a clinician-determined inspiratory pressure. Cycle variables are generally a set volume or a set inspiratory time. Breaths can also be cycled if pressure limits are exceeded. The four common breath types supplied by modern mechanical ventilators to provide total support are volume control (VC), volume assist (VA), pressure control (PC), and pressure assist (PA).3 These breaths are classified by their trigger, target, and cycle features in Figure 48-1.
Mode Controller
The availability and delivery logic of different breath types define the mode of mechanical ventilatory support.3 The mode controller is an electronic, pneumatic, or microprocessor-based system designed to provide the proper combination of breaths according to set algorithms and feedback data (conditional variables). For total support, the most commonly used modes are volume assist-control and pressure assist-control. Synchronized intermittent mandatory ventilation (SIMV) can provide VA and VC or PA and PC breaths interspersed with either unsupported or partially supported spontaneous breaths (volume-targeted SIMV and pressure-targeted SIMV, respectively). When the SIMV machine breath rate is set sufficiently high, the bulk of the work required for the desired delivered minute ventilation is borne by the ventilator such that these modes can be considered to provide virtual total support. A variation on the SIMV approach is to use a pressure-targeted mode with a long inspiratory time/short expiratory time pattern and allow spontaneous breaths to occur during the long inflation phase. This approach goes by a variety of proprietary names but is most commonly referred to as airway pressure release ventilation (APRV).4 These modes are summarized according to available breath types in Table 48-1.
New ventilator designs incorporate advanced monitoring and feedback functions into these controllers to allow continuous adjustments in mode algorithms as the patient’s condition changes.5 The most common of these new feedback designs is the addition of a volume target backup to pressure assist-control, termed pressure-regulated volume control (PRVC). This feature adjusts the inspiratory pressure level above or below the clinician-set target to achieve the volume target. A more sophisticated feedback system for pressure-targeted breaths calculates a frequency–tidal volume combination that requires the least ventilator work for the desired minute ventilation. Known as adaptive support ventilation (ASV), this mode also incorporates a calculation of the expiratory time constant to assure that an expiratory time to minimize air trapping is also present.6 Finally, two new modes that are driven entirely by patient effort can be set to provide virtually all the work of breathing and thus could be considered forms of total support. One is proportional assist ventilation (PAV), which drives ventilator gas flow as a proportion of patient flow demand; the other is neurally adjusted ventilator assistance (NAVA), which drives ventilator gas flow as a proportion of the diaphragmatic electromyogram signal.6–8 These two interactive modes are discussed in more detail in Chapter 49.
Other Device Features Supporting Mechanical Ventilation
Effort sensors are pressure and/or flow transducers in the ventilator circuitry that detect patient breathing efforts and are characterized by their sensitivity and responsiveness.9 Blenders mix air and O2 to produce a delivered inspired O2 fraction (FIO2) from 0.21 to 1.0. On newer systems, blenders are also available for other gases such as heliox, nitric oxide, and anesthetic agents. Humidifiers adjust blended gas mixtures to approximate body conditions using either passive heat-moisture exchangers in the circuitry or active systems that add heat and moisture directly. Positive end-expiratory pressure (PEEP) is usually applied by regulating pressure in the expiratory valve of the ventilator system, but a continuous flow of source gas during the expiratory phase can produce a similar effect. The gas delivery circuit consists of flexible tubing that often has pressure or flow sensors and an exhalation valve. It is important to remember that this tubing has measurable compliance (generally 1–4 mL/cm H2O), and significant amounts of delivered gas may only distend this circuitry rather than enter the patient’s lungs when high airway pressures are encountered.
 Physiologic Effects of Positive-Pressure Mechanical Ventilation
Physiologic Effects of Positive-Pressure Mechanical Ventilation
Equation of Motion
Lung inflation during mechanical ventilation occurs when pressure and flow are applied at the airway opening. These applied forces interact with respiratory system compliance (both lung and chest wall components), airway resistance, and to a lesser extent, respiratory system inertance and lung tissue resistance to effect gas flow.10,11 For simplicity’s sake, because inertance and tissue resistance are relatively small, they can be ignored, and the interactions of pressure, flow, and volume with respiratory system mechanics can be expressed by the simplified equation of motion:
In a mechanically ventilated patient, this relationship is expressed as:
Separating chest wall and lung compliance (CCW and CL, respectively) during a passive, machine-controlled positive-pressure breath requires an esophageal pressure measurement (Pes) to approximate pleural pressure. With this measurement, the inspiratory change in Pes (dPes) can be used in the following calculations: CCW = VT/dPes, and CL = VT/(dPAO − dPes). In clinical practice, because CCW is usually quite high and dPes is thus quite low, dPAOplateau and PAOplateau are often taken as an approximation of lung distending pressure. However, in situations in which CCW is reduced (e.g., obesity, anasarca, ascites, surgical dressings), the stiff chest wall can have a significant effect on dPAOplateau and PAOplateau and must therefore be considered when using these measurements to assess lung stretch.12
Patient-Ventilator Interactions and Synchrony
During the assisted breaths of assist-control ventilation, patients interact with all three phases of breath delivery: trigger, target, and cycle.13 As noted, breath triggering occurs when patient effort is sensed by the ventilator and flow delivery is initiated. Breath triggering is characterized by sensitivity (the amount of effort required to trigger the breath) and responsiveness (the time required to have flow delivery meet the target value). Once flow delivery is initiated, ventilator flow delivery interacts with patient flow demand. Flow synchronized to demand is characterized by an airway pressure profile that is similar in shape to a controlled breath. Ventilator breath cycling that is synchronous to patient effort is characterized by a smooth transition in the airway pressure and flow graphic from inspiration to expiration.
Respiratory System Mechanics and Breath Design Features
As noted earlier, there are two basic approaches to delivering positive-pressure breaths during assist-control ventilation: pressure targeting–time cycling and flow targeting–volume cycling. Although similar ranges of tidal volume and inspiratory time are available with either strategy, these breath characteristics interact differently with changing respiratory system mechanics and patient effort.10,11 Changes in compliance or resistance cause a change in tidal volume (but not in pressure at the airway opening) with a pressure-targeted breath. In contrast, similar changes in compliance or resistance cause a change in pressure at the airway opening (but not in flow or volume) with a flow-targeted breath. Patient effort during a pressure-assist breath causes the ventilator to augment flow (and thus volume) to maintain the inspiratory pressure target; this same effort during a volume-assist breath does not affect delivered flow or volume but instead causes a fall in the measured circuit pressure. The hybrid breath design pressure-regulated volume control described earlier has basic features of pressure targeting but also has a volume feedback feature that adjusts the pressure target to maintain a clinician set volume.
Intrinsic Positive End-Expiratory Pressure and the Ventilatory Pattern
Intrinsic PEEP develops within the alveoli because of inadequate expiratory time or collapsed airways during expiration (or both). Intrinsic PEEP depends on three factors: minute ventilation, the expiratory time fraction, and the respiratory system’s expiratory time constant (the product of resistance and compliance).14 As minute ventilation increases, expiratory time fraction decreases, or time constant lengthens (i.e., higher resistance or compliance values), the potential for intrinsic PEEP to develop increases.
Distribution of Ventilation
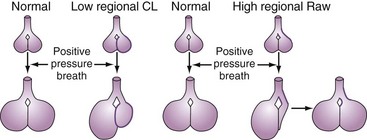
A positive-pressure tidal breath must distribute itself among the millions of alveolar units in the lung.15,16 Factors affecting this distribution include regional resistances, compliances, and functional residual capacities and the delivered flow pattern (including inspiratory pause). In general, positive-pressure breaths tend to distribute more to units with high compliance and low resistance and away from obstructed or stiff units (Figure 48-2). This creates the potential for regional overdistention of healthier lung units, even in the face of “normal-sized” tidal volumes.
 Alveolar Recruitment
Alveolar Recruitment
Infiltrative lung disease produces severe (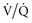 ) mismatching through alveolar flooding and collapse.17 In many (but not all) of these disease processes, the collapsed alveoli can be recruited during a positive-pressure ventilatory cycle.18,19 Three specific techniques to optimize recruitment are the application of PEEP, use of recruitment maneuvers, and prolongation of inspiratory time.
) mismatching through alveolar flooding and collapse.17 In many (but not all) of these disease processes, the collapsed alveoli can be recruited during a positive-pressure ventilatory cycle.18,19 Three specific techniques to optimize recruitment are the application of PEEP, use of recruitment maneuvers, and prolongation of inspiratory time.
PEEP is defined as an elevation of transpulmonary pressures at the end of expiration.18–20 As discussed, PEEP can be produced either by expiratory circuit valves (applied PEEP) or as a consequence of ventilator settings interacting with respiratory system mechanics (intrinsic PEEP). Note that expiratory muscle contraction can also raise intrathoracic pressures at end-expiration; this should not be considered PEEP, however, because it is not a transpulmonary pressure (i.e., alveolar-pleural pressure).
Alveoli that are prevented from “derecruiting” by PEEP provide several potential benefits. First, recruited alveoli improve (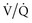 ) matching and gas exchange.18–21 Second, as discussed in more detail later, patent alveoli throughout the ventilatory cycle are not exposed to the risk of injury from the shear stress of repeated opening and closing.22 Third, PEEP prevents surfactant breakdown in collapsing alveoli and thus improves lung compliance.23
) matching and gas exchange.18–21 Second, as discussed in more detail later, patent alveoli throughout the ventilatory cycle are not exposed to the risk of injury from the shear stress of repeated opening and closing.22 Third, PEEP prevents surfactant breakdown in collapsing alveoli and thus improves lung compliance.23
PEEP can also be detrimental. Because the tidal breath is delivered on top of the baseline PEEP, end-inspiratory pressures are raised by PEEP application.24 This must be considered if the lung is at risk for stretch injury (see Ventilator-Induced Lung Injury). Moreover, because alveolar injury is often quite heterogeneous, appropriate PEEP in one region may be suboptimal in another region and excessive in another. Optimizing PEEP is thus a balance between recruiting the recruitable alveoli in diseased regions without overdistending already recruited alveoli in healthier regions. Another potential detrimental effect of PEEP is that it raises mean intrathoracic pressure. This can compromise cardiac filling in susceptible patients (see Cardiac Effects).
Recruitment maneuvers are based on the concept that alveolar recruitment occurs throughout a positive-pressure inflation—all the way to total lung capacity.25 In practice, recruitment maneuvers are performed using sustained inflations (e.g., 30 to 40 cm H2O for up to 2 minutes).25–27 An alternative approach is to use frequent “sigh breaths” that briefly take the lung to near total capacity on a frequent basis.28 It must be pointed out that recruitment maneuvers provide only initial alveolar recruitment; the duration of recruitment almost certainly depends on an appropriate setting of PEEP to prevent subsequent derecruitment.27
Prolonging the inspiratory time (generally by adding a pause), often used in conjunction with a rapid-decelerating flow (i.e., pressure-targeted) breath, has several physiologic effects.29,30 First, the longer inflation period may lead to the opening of more slowly recruitable alveoli. Second, increased gas mixing time may improve (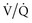 ) matching in infiltrative lung disease. Third, the development of intrinsic PEEP can have similar effects to that of applied PEEP (see earlier). Indeed, much of the improvement in gas exchange associated with long inspiratory time strategies may be merely a PEEP phenomenon.30 It should be noted, however, that the distribution of intrinsic PEEP (most pronounced in lung units with long time constants) may be different from that of applied PEEP; thus, (
) matching in infiltrative lung disease. Third, the development of intrinsic PEEP can have similar effects to that of applied PEEP (see earlier). Indeed, much of the improvement in gas exchange associated with long inspiratory time strategies may be merely a PEEP phenomenon.30 It should be noted, however, that the distribution of intrinsic PEEP (most pronounced in lung units with long time constants) may be different from that of applied PEEP; thus, (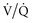 ) effects may also be different.31 Fourth, because these long inspiratory times significantly increase total intrathoracic pressures, cardiac output may be affected (see Cardiac Effects). Finally, inspiratory-expiratory ratios that exceed 1 : 1 (so-called inverse ratio ventilation [IRV]) are uncomfortable, and patient sedation or paralysis is often required unless a relief mechanism allows spontaneous breathing during the inflation period (airway pressure release ventilation; see later).
) effects may also be different.31 Fourth, because these long inspiratory times significantly increase total intrathoracic pressures, cardiac output may be affected (see Cardiac Effects). Finally, inspiratory-expiratory ratios that exceed 1 : 1 (so-called inverse ratio ventilation [IRV]) are uncomfortable, and patient sedation or paralysis is often required unless a relief mechanism allows spontaneous breathing during the inflation period (airway pressure release ventilation; see later).

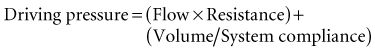
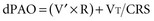
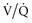 ) matching (e.g., a more uniform ventilation distribution may actually worsen (
) matching (e.g., a more uniform ventilation distribution may actually worsen (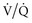 ) matching in a lung with inhomogeneous perfusion). Because of all these considerations, predicting which flow pattern will optimize
) matching in a lung with inhomogeneous perfusion). Because of all these considerations, predicting which flow pattern will optimize 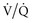 matching is difficult and often an empirical trial-and-error exercise.
matching is difficult and often an empirical trial-and-error exercise.




