Chemical structure of an ester (procaine) and an amide (lidocaine)
Local anaesthetic agents exist in two states, acid (protonated) and basic (non-ionised) in equilibrium according to their pKa and ambient pH, as determined by the Henderson–Hasselbalch equation:
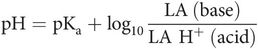
Local anaesthetic agents are weak bases. At physiological pH, there exists a mixture of non-ionised and ionised drug. This is important, as only the non-ionised drug passes through the membrane, yet it is only the ionised drug that is active. Small changes in pH have marked effects on the proportion of drug that is ionised, and therefore markedly influence the effect.
Mechanism of action
Injectable local anaesthetics must be soluble and stable in water. This is achieved by creating hydrochlorides of the drug. Hydrochlorides are soluble in water and produce an acid environment with a high degree of ionisation.
Local anaesthetic agents act by blocking the fast sodium channel in neuronal membranes. To do so the drug must be in the protonated form and the ion channel must be in the open state. The drug enters the ion channel from the intracellular direction, but is administered extracellularly. Blockade by this route is use-dependent, because ionophores are only blocked while open.
The process by which local anaesthetic agents reach the sodium channel is illustrated in Figure 33.2, using lidocaine as an example:
1. The pKa of lidocaine is 7.9, so extracellularly (at pH 7.4) 24% is in the non-ionised state and 76% in the ionised state.
2. The non-ionised drug is therefore relatively lipophilic, and it passes passively down the concentration gradient through the membrane into the cell.
3. Intracellularly, pH is about 7.1, and this shifts the balance of ionisation of the intracellular portion of drug towards the ionised state (86%).
4. The ionised drug, attracted by the negative charge of membrane protein, then passes into the open ion channel.
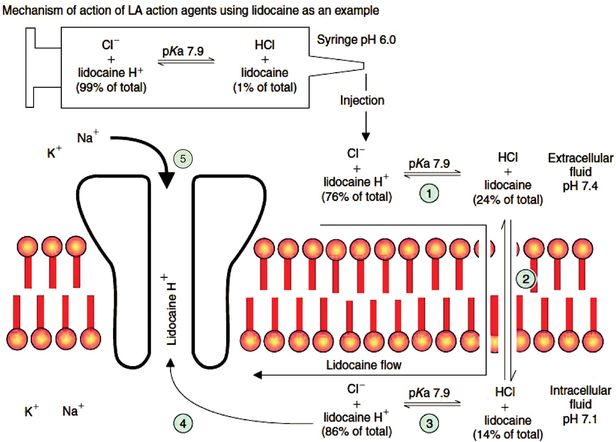
Mechanism of action of local anaesthetic agents, using lidocaine as an example. The stages indicated by circled numbers are described in more detail in the text.
Another mechanism of action may contribute to the anaesthetic activity. This involves the passage of non-ionised drug through the membrane directly blocking the sodium channel, an effect that does not rely on the sodium channel being open.
The opening of the fast sodium channels in neuronal membranes and the passage of sodium through them are essential for the development and propagation of the action potential. The sharp upstroke of the action potential is gradually attenuated as more sodium channels become blocked. When the depolarisation is insufficient to generate the currents required to depolarise neighbouring membrane, then action-potential propagation and neuronal transmission cease.
Factors influencing activity
Figure 33.3 shows the pharmacological properties of important local anaesthetic agents.
| Molecular weight (Da) | pKa (25 °C) | Partition coefficient (heptane buffer) | Protein binding (%) | Onset | Potency (relative to lidocaine) | Duration | |
|---|---|---|---|---|---|---|---|
| Esters | |||||||
| Amethocaine | 264 | 8.5 | 4.1 | 76 | Slow | 4 | Long |
| Procaine | 236 | 8.9 | 0.02 | 6 | Slow | ½ | Short |
| Amides | |||||||
| Bupivacaine | 288 | 8.1 | 27.5 | 96 | Medium | 4 | Long |
| Lidocaine | 234 | 7.9 | 2.9 | 64 | Rapid | 1 | Medium |
| Prilocaine | 220 | 7.9 | 0.9 | 55 | Rapid | 1 | Medium |
| Ropivacaine | 274 | 8.1 | 6.1 | 95 | Medium | 4 | Long |
Molecular weight
Molecular weight itself does not affect the pharmacological properties. However, increases in molecular weight tend to be indicative of increased side-chain size and therefore increased lipid solubility.
Lipid solubility
The higher the lipid solubility, the greater the penetration of the nerve membrane by the drug, so that higher lipid solubility results in greater potency. It also results in more toxicity and local irritancy. High lipid solubility also increases the rate of onset and duration of action of local anaesthetic agents.
pKa
The lower the pKa, the lower the degree of ionisation for any given pH and so the more rapid the speed of onset of the block. Increasing pKa increases the ionised proportion of the drug so that intracellularly a higher proportion is in the active state. However, this also means that less is in the non-ionised, diffusible state, so the onset and offset of action are also slower. Figure 33.4 shows the effect of differences in pH and pKa on the proportion of ionised and non-ionised local anaesthetic agents.
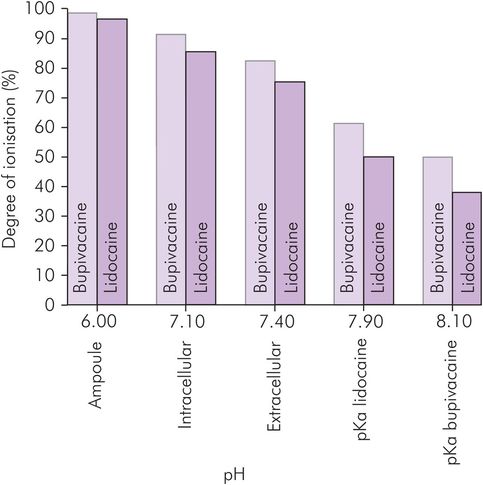
Effect of pH on the ionisation of local anaesthetic agents
pH
Acidosis (low pH) increases the proportion of ionised drug in the interstitium, and therefore reduces the amount of drug able to cross the neuronal membrane. The local anaesthetic is therefore reduced in potency. Manipulation of the pH of the local anaesthetic solution by addition of alkali, buffers or carbonation may be used to alter the proportion of non-ionised drug.
Protein binding
The degree of protein binding reflects the ability of the drug to bind to membrane proteins: the greater the binding, the longer the duration of action. Increased binding to tissue protein correlates with an increase in the duration of action and probably indicates a higher affinity for membrane proteins (for example, the fast sodium ionophore).
Ester-linked agents
Amethocaine
Amethocaine (tetracaine) is an ester local anaesthetic agent used for topical anaesthesia. It is available as a gel (4%) for local anaesthesia of the skin before intravascular cannulation. Applied to the skin, it is effective within 45 minutes. It can then be removed, and remains active for 4–6 hours. The preparation should not be applied to inflamed or damaged skin or highly vascular tissues, as it is rapidly absorbed through mucosal surfaces. More dilute solutions (0.5% and 1%) are available for topical anaesthesia of the conjunctiva. Amethocaine is potent and readily absorbed, but in common with other ester local anaesthetics it may cause hypersensitivity.
Benzocaine
Benzocaine is an unusual ester local anaesthetic agent in that the side chain is an ethyl group with no amine component, and therefore remains un-ionised. Benzocaine has a low potency but it may cause methaemoglobinaemia. It is a component of some throat lozenges, and may be applied directly to painful skin ulcers.
Cocaine
Cocaine is a naturally occurring ester derived from benzoic acid, and extracted from the leaves of Erythroxylum coca. It is available in solution and pastes in concentrations ranging from 1% to 10%. It is mainly used for topical anaesthesia and to reduce bleeding during nasal surgery. Cocaine is rapidly taken up into mucous membranes to provide anaesthesia and intense vasoconstriction. This vasoconstriction limits systemic absorption, resulting in a bioavailability by this route of 0.5%. It is well known as a drug of abuse with high addictive potential, and in this role is taken by chewing, inhaling nasally, smoking or intravenously. It causes marked sympathomimetic activity, and arrhythmias are a definite risk. Systemic injection must be avoided. Systemically absorbed cocaine has a volume of distribution of 2 L kg–1, with 98% being protein-bound. It is eliminated by plasma and liver esterases, having a clearance of 35 mL kg–1 min–1 and a half-life of 45 minutes.
Cocaine shares the same mechanism of action as the other local anaesthetic agents (pKa = 8.7), and also inhibits catecholamine neuronal uptake-1. Synaptic levels of dopamine and noradrenaline increase, resulting in the central stimulation and euphoria, and vasoconstriction. Cocaine initially blocks the inhibitory pathways, resulting in euphoria, hyperthermia, altered vision and hearing, nausea and eventually convulsions. Higher levels of cocaine also block the excitatory pathways, resulting in central nervous depression leading to sedation and unconsciousness, with respiratory depression. Cocaine causes mydriasis and raised intraocular pressure. It is no longer used for local anaesthesia of the eye. The central stimulation increases respiratory rate and volume. A rise in sympathetic tone leads to tachycardia and hypertension, the latter being exacerbated by peripherally mediated vasoconstriction. High doses depress the myocardium. The general excitation also increases metabolic rate, which contributes to the hyperthermia and raises oxygen consumption and CO2 production. The recommended maximum dose is 1.5 mg kg–1. Cocaine should be avoided in porphyria.

Full access? Get Clinical Tree







