Chapter 95 Life-Threatening Viral Diseases and Their Treatment
Myocarditis
Background
Although many infectious and noninfectious causes have been identified, viruses account for most cases of myocarditis.1 The spectrum of disease ranges from asymptomatic, with only minimal changes on the electrocardiogram, to fulminant, with rapid onset of severe disease. Likewise, myocardial involvement may be focal or diffuse.2 Most patients have an indolent illness that may progress to dilated cardiomyopathy. Because of the varied presentations and the difficulty in the establishment of a definitive diagnosis, the true incidence of myocarditis is unknown. In a large series from Sweden, 1% of myocardial biopsies from autopsies conducted over a 10-year period fulfilled the Dallas criteria3 for myocarditis.4
Pathogenesis
Although the pathogenesis of viral myocarditis is not well understood, viruses appear to enter cardiac myocytes or macrophages through specific receptors and coreceptors.5 Viral virulence is likely modified by differential coreceptor binding6 and variations in the viral genome.7 Myocardial damage is thought to occur at least in part as a direct result of viral infection, with active viral replication leading to myocardial necrosis.8 Coxsackie virus protease 2A cleaves dystrophin in cultured myocytes and in infected mouse hearts. The results are impaired dystrophin function and poor myocyte contractility.9 In addition, both humoral and cellular immune responses contribute to the pathogenesis of myocarditis,10,11 through postinfectious autoimmune processes,11 cytotoxic T lymphocytes, and antibody-dependent cell-mediated cytotoxicity.12 Cytokines13 may also cause direct myocardial injury and affect cardiac function.
Cause
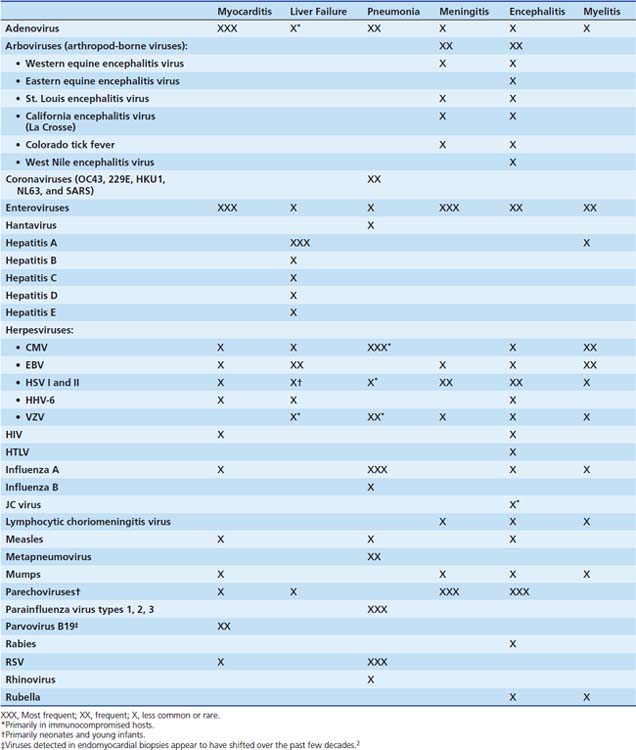
The viruses most frequently associated with myocarditis are enteroviruses, particularly Coxsackie virus B, and adenoviruses (serotypes 2 and 5).14 Many other viruses have caused myocarditis in children, including influenza A, herpes simplex virus (HSV); human immunodeficiency virus (HIV); cytomegalovirus (CMV); respiratory syncytial virus (RSV); and the mumps and measles viruses, before the widespread use of the measles-mumps-rubella (MMR) vaccine (Table 95-1). Polymerase chain reaction (PCR) of cardiac tissue from endomyocardial biopsy specimens in 34 children with a clinical diagnosis of myocarditis identified adenovirus in 44%, enterovirus in 24%, and HSV in 6%.15 In addition to enteroviruses, adenovirus,14 CMV, human herpesvirus 6 (HHV-6) and parvovirus B1916 are increasingly recognized as important causes of myocarditis in adolescents and adults. Adenoviruses and enteroviruses are the viruses most frequently identified in patients with dilated cardiomyopathy.
Clinical Presentation
Infants with myocarditis usually have symptoms that include poor feeding, fever, irritability, and listlessness. Physical findings are consistent with congestive heart failure. Enteroviral myocarditis in infancy frequently occurs in conjunction with severe hepatitis, pneumonitis, or both and can be difficult to distinguish from bacterial sepsis.17 The death rate may be as high as 75%. Severe dysrhythmias have been described in infants with myocardial involvement from RSV,18 and viral myocarditis has been implicated in some cases of sudden death.19,20 Older children and adolescents are more likely to appear for examination after a prodromal viral illness. Early symptoms include lethargy, low-grade temperature, and decreased appetite. They may have diaphoresis, dyspnea on exertion, malaise, chest pain, and palpitations. Resting tachycardia disproportionate to the amount of fever is common, and an apical systolic murmur may be heard. A subset of children and adults have fulminant myocarditis, characterized by rapid onset of symptoms, severe hemodynamic compromise, and fever.21
Laboratory abnormalities may include elevated white blood cell count and erythrocyte sedimentation rate.22 Serum aspartate aminotransferase (AST) levels are often elevated,23 as are creatinine kinase-MB levels. Cardiac troponin I may be a more sensitive measure of cardiac muscle injury in myocarditis.24
Electrocardiographic abnormalities are almost always present in acute myocarditis, with findings of low-voltage QRS complexes and nonspecific ST and T wave changes. Both atrial and ventricular arrhythmias may be present, including supraventricular and ventricular tachycardia, as well as conduction abnormalities. Echocardiography reveals left ventricular dysfunction, in most cases with either segmental wall motion abnormalities or global hypokinesis. Pericardial effusions are common. In one series, nondilated, thickened, and hypocontractile left ventricles (LVs) were seen in subjects with fulminant myocarditis compared with significant LV dilation and normal LV thickness in subjects with a more insidious onset. Subjects with fulminant myocarditis had more evidence of inflammation on endomyocardial biopsy and were more likely to recover ventricular function.25 Pulmonary edema, enlarged cardiac silhouette, and prominent pulmonary vasculature may be seen on a chest radiograph. Contrast-enhanced cardiac magnetic resonance imaging (MRI) can document the location and extent of inflammation and can be used to assist in diagnosis or to guide endomyocardial biopsy 26,27
Acute Liver Failure
Background
Acute liver failure (ALF) is a rare condition, which prior to the availability of orthotopic liver transplantation, carried a mortality of 70% to 80% (see Chapter 88). Early studies of ALF in children used the adult definition that required rapid development of severe hepatic dysfunction with the development of hepatic encephalopathy within 8 weeks of clinical jaundice in a person without a prior history of liver disease. However, due to the difficulty in assessing encephalopathy in young children and the recognition that terminal liver failure can occur in the absence of clinical encephalopathy, the Pediatric Acute Liver Failure Study (PALF) group has accepted the diagnosis of ALF in children with no history of chronic liver disease who present with biochemical evidence of acute liver injury and severe hepatic-based coagulopathy regardless of the presence or absence of hepatic encephalopathy.28 The causes of ALF in children can be metabolic, toxic, drug-related, immune-mediated or infectious. The percentage of ALF caused by viral infections varies significantly by age group and geographic location, with infections causing only 6% of ALF in a large pediatric series from North America and Great Britain28,29 but 50% in a series from South America.30
Cause
While less than 1% of infections with these viruses result in ALF, the hepatotropic viruses, hepatitis A and B, comprise the majority of cases with a definitive viral diagnosis, particularly in endemic areas.30–34 Infection with hepatitis C rarely causes ALF; however, there are case reports of ALF with both postnatally and perinatally acquired hepatitis C in children.28,35,36 In 2006, PALF published a report of the first 348 children presenting with ALF to the study sites throughout North America and Great Britain. Only 6% of the cases in this series had definitive viral causes, with the viruses identified being adenovirus, CMV, hepatitis A, enterovirus, HSV, Epstein-Barr virus (EBV), and hepatitis C. However, both in this series and in a later series of 703 children published by the same group, almost 50% of all the cases were indeterminate, due at least in part to lack of a complete diagnostic workup.29 It is likely that some of these indeterminate cases were also due to undiagnosed infectious causes. Additional viruses that have been implicated in ALF include hepatitis D and E, parvovirus,37,38 varicella-zoster virus (VZV),39 and HHV-6.40,41 ALF in infants is most likely to be associated with systemic illness due to enterovirus, echovirus, HSV, HHV-6, or CMV.28,42–44 While most perinatally acquired infections with hepatitis B are asymptomatic, infants born to women with both HBsAg and anti-HBeAb appear to be at greater risk for ALF due to perinatally acquired hepatitis B.45
Risk factors for HSV hepatitis outside of the neonatal period include pregnancy and immune suppression.46,47 Immune suppression is also a risk factor for CMV-, adenovirus-, and VZV-associated ALF. Hepatitis E virus (HEV) is an enterically transmitted virus that causes epidemic hepatitis in many areas of the world, particularly the Indian subcontinent and Southeast Asia.34 ALF can also occur in severe dengue48,49 and yellow fever infection.48–50 HEV, dengue, and yellow fever are not endemic in Western countries.
Clinical Presentation
Symptoms of acute hepatitis include jaundice, anorexia, fatigue, nausea, and vomiting.32,51,52 In fulminant disease, there is rapid progression to hepatic failure and encephalopathy. Physical examination may demonstrate fever, hepatosplenomegaly with liver tenderness, scleral or cutaneous icterus, and mucosal bleeding. Patients with severe vomiting may have significant dehydration. Laboratory studies include elevated hepatic enzymes (10- to 100-fold increases in AST and alanine aminotransferase), hyperbilirubinemia, prolonged prothrombin time, and elevated ammonia levels. As hepatocyte necrosis progresses, hepatic enzyme levels and liver size may decrease. Cerebral edema is common in patients with severe encephalopathy32,52 and renal failure is a common complication.51,52
Viral Pneumonia/Pneumonitis
Background
Influenza and pneumonia combined are a leading cause of death of children in developing countries and the eighth leading cause of death in the United States for patients of all ages. A greater burden of disease is present in infants, young children, and older individuals.53 Although only 20% to 50% of community-acquired pneumonias in adults are associated with viral pathogens,54–58 viruses account for the majority of the causes of lower respiratory infection in children. It is estimated that more than 500,000 hospitalizations occur each year in the United States for lower respiratory tract infections among children younger than 18 years old,59,60 and an estimated 80% of these hospitalizations are attributable to viral etiologies, with the majority in children aged less than 5 years.59,61 The peak season is from midwinter to early spring.
Cause
The etiologic agents of viral pneumonia are varied (see Table 95-1), and recent studies using PCR for diagnosis have considerably improved the ability to detect viruses. RSV is the primary cause of hospitalization for respiratory tract illness in young children, with average annual hospitalization rates in the United States of 17 per 1000 children under 6 months of age and 3 per 1000 children younger than 5 years.62 In a recent national surveillance study, most children with RSV infection had no coexisting medical conditions or characteristics that significantly identified them as being at greater risk for severe RSV disease, except for being younger than 2 years.62 Among children, RSV infection is the cause of 50% to 90% of hospitalizations for bronchiolitis, 5% to 40% of those for pneumonia, and 10% to 30% of those for tracheobronchitis.63 Repeat infections are common; in the healthy host, they are localized to the upper respiratory tract. Among immunocompromised patients, RSV upper respiratory infections can progress to fatal pneumonia, with the greatest mortality risk in patients with severe lower tract disease and in those in whom treatment is significantly delayed.64,65
Influenza epidemics occur annually with significant morbidity and death in young children and older individuals. In a national surveillance study, an average of 0.9 per 1000 children aged 0 to 59 months were hospitalized with laboratory-confirmed influenza, with the highest rate of 4.5 per 1000 children in the 0 to 5-month age group.66 During the four influenza seasons from 2003 to 2007, the total number of reported pediatric influenza deaths ranged from 46 to 153, with an average of 82 deaths each year.67 Infected infants younger than 2 months may have symptoms mimicking bacterial sepsis, commonly including apnea. In children younger than 5 years, influenza can cause symptoms of laryngotracheobronchitis, whereas pneumonia occurs in 10% to 15% of those younger than 3 years. Finally, older children generally exhibit the classic flulike symptoms of fever, headache, myalgia, and malaise with upper respiratory tract symptoms. Bacterial superinfection is a common and potentially severe complication of influenza. Among immunocompromised patients, risk factors for more severe disease and progression to the lower respiratory tract include lymphopenia and infection early after hematopoietic stem cell transplant.65, 68 With earlier antiviral treatment, progression to the lower tract and mortality have been markedly reduced.
In 2009, the emergence of a pandemic strain of influenza A, termed novel H1N1, led to a greatly increased burden of influenza disease worldwide. Children and young adults were at a disproportionate risk for infection and hospitalization, with 60% of infections occurring among those 18 years of age or younger, and 95% in those younger than 50 years.69–71 The overall attack rate was highest among children aged 5 to 14 years (147 per 100,000 population),69 but infants had the highest hospitalization rates and persons aged 50 years or older had the highest mortality rates once hospitalized.69,71 This differs from seasonal influenza, where about 60 percent of influenza-related hospitalizations and 90 percent of influenza-related deaths occur in people 65 years and older. From April to October of 2009, the Centers for Disease Control and Prevention (CDC) received reports of 145 pediatric deaths associated with influenza infection.67 Correcting for under-ascertainment, the CDC estimates the actual count may be three to four times greater than reported (http://www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm). The majority of hospitalized cases had underlying medical conditions associated with severe seasonal influenza; secondary bacterial infections were observed in 15% to 30% of fatal cases.71,72
In a typical influenza season, parainfluenza viruses (PIVs) are generally second only to RSV as important viral causes of lower respiratory infection in children and immunocompromised patients.73–77 The average annual PIV hospitalization rate is 1.02 per 1000 children 0 to 59 months of age, with the highest rate of 3.01 per 1000 in children 0 to 5 months of age.77 PIVs account for 50% of hospitalizations for acute laryngotracheitis (croup) and at least 15% of cases of bronchiolitis and pneumonia. PIVs types 1 and 2 cause more cases of croup whereas PIV type 3 is more likely to infect the small air passages and cause pneumonia or bronchiolitis. However, any PIV can cause lower respiratory tract disease, particularly during primary infection or in immunosuppressed patients. In immunocompromised hosts, virologically confirmed PIV pneumonia has a 30-day attributable mortality rate of at least 30% to 35%.75,78,79 Unlike RSV lower tract disease, in which RSV is usually the single pathogen, copathogens may be identified with PIV pneumonia more than 50% of the time,75 and management of PIV pneumonia should include workup and treatment for copathogens.
Other respiratory viruses that can cause pneumonia, particularly in young children and immunocompromised hosts, include human metapneumovirus, adenovirus, human rhinoviruses (HRVs), and human coronaviruses (HCoVs). Since it was first described in 2001,80 human metapneumovirus has been shown to be a common cause of croup, bronchiolitis, and pneumonia in children, the elderly, and immunocompromised patients.81–89 The clinical manifestations of human metapneumovirus are indistinguishable from RSV. Adenovirus pneumonia can occur as an isolated event or as part of disseminated disease. Risk factors for adenovirus pneumonia include a compromised immune function, chronic underlying respiratory or cardiac disease, and age younger than 7 years.90 Adenovirus pneumonia due to specific serotypes has been associated with outbreaks among young children (types 3 and 7),91 high school students (type 11),92and a recent community-based outbreak that included children (type 14).93 HRVs occasionally cause lower respiratory tract disease requiring admission to intensive care among pediatric and immunosuppressed patients, although a causative role is sometimes difficult to define because HRVs frequently occur in association with copathogens.94–98 Among pediatric patients, a new species of rhinovirus (HRV-C) has been discovered that may cause more severe disease than HRV-A or HRV-B, although this association is not yet clearly elucidated.99–104
The HCoV family currently includes five viruses that are known to infect humans: HCoV-229E, HCoV-OC43, the severe acute respiratory syndrome-associated CoV (SARS-CoV), and the recently described HCoV-NL63 and HCoV-HKU1. The SARS outbreak originated in Guangdong Province in China in the fall of 2002 and was characterized by a life-threatening, atypical pneumonia caused by a novel coronavirus most likely derived from masked palm civets.105 SARS-CoV was spread by close contact with infected humans, mostly to household contacts and health care workers. Death from progressive respiratory failure occurred in approximately 10% to 15% of adult patients106–110; in children, morbidity was less and no deaths occurred.111–113 SARS-CoV is not currently circulating in the world. The most recent human cases of SARS-CoV infection were reported in China in April 2004 in an outbreak resulting from laboratory-acquired infections.114 Should SARS-CoV re-emerge, updates to the case definition, including clinical criteria for moderate or severe respiratory illness of unknown cause and epidemiologic criteria for exposure, can be found at www.cdc.gov/SARS. Other HCoVs have been reported to cause pneumonia in children and immunocompromised patients treated for hematologic malignancies.115–119
Additional information about respiratory viruses including RSV, influenza, PIV, and adenovirus is available in Chapter 47.
Although CMV usually causes relatively benign disease in immunocompetent hosts, it is frequently severe and often fatal in immunocompromised hosts, including patients with acquired immunodeficiency syndrome (AIDS), malignancy, congenital immune deficiencies, and transplant recipients. CMV is a herpesvirus and, like other herpesviruses, it can cause latent infection in vascular endothelial cells, monocytes and macrophages, polymorphonuclear neutrophils, and renal and pulmonary epithelial cells. Cellular damage is caused directly by the viral lytic infection or indirectly by the immune response of the host. Among allogeneic stem cell transplant recipients, the risk of CMV pneumonia is high; historically, treatment of CMV pneumonia with ganciclovir and intravenous immunoglobulin (IVIG) in the late 1980s dramatically reduced mortality from 80% to 100% without therapy to approximately 50%.120 The spectrum of CMV pneumonia has changed with the introduction of routine antiviral prophylaxis and preemptive therapy strategies.121 As a result, CMV disease during the first 3 months after hematopoietic cell transplantation has been reduced from 20% to 30% to less than 5% in most studies.122 CMV disease now primarily occurs late after transplant. Risk factors for late CMV disease include reactivation of (or primary infection with) CMV during the early period after transplant, and therapy for acute or chronic graft-versus-host disease.120 Among solid organ transplant recipients, the risk of CMV disease is greatest for lung transplant recipients, followed by liver, heart, and renal transplant recipients.120,123
Hantaviruses are known for causing hemorrhagic fevers and acute severe respiratory infection in young adults. Hantaviruses can spread from mammal to mammal, including humans, by exposure to aerosolized feces, infected urine, or other secretions. In the United States, the Sin Nombre virus, which causes the pulmonary syndrome, is found in 10% to 80% of deer mice in rural areas of North America, although the overall seroprevalence rates in the western United States are less than 0.1%.124 In the United States, hantaviral infections are rare in children under the age of 10 years; however, severe cases resulting in death have been described in children as young as 5 years old in South America.124,125 Hantaviruses cause disease by creating leakage of plasma and erythrocytes through the vascular endothelium in the lung (hantavirus pulmonary syndrome [HPS]) or the kidneys (hemorrhagic fever with renal syndrome). The differential diagnosis includes influenza A, rickettsial disease, borreliosis, tularemia, Legionella pneumophila, Chlamydia pneumoniae, Mycoplasma pneumoniae and Pneumocystis jiroveci. HPS is fatal in 35% to 40% of cases across the Americas.124,126 Supportive care, including early consideration of extracorporeal membrane oxygenation, is key to treatment of patients with HPS.
Central Nervous System Infections
Background
Aseptic meningitis, encephalitis, and myelitis are inflammatory conditions of the central nervous system (CNS), involving meninges, brain, and spinal cord, respectively. Disease is caused by a variety of infectious pathogens, but viruses cause most disease. Viruses gain entry to the CNS via the bloodstream (enteroviruses and arboviruses) or by direct neuronal spread (HSV and rabies). Pathogenesis may involve direct viral invasion or a vigorous virus-specific immune response resulting in damage to the neurons and supporting cells. Alternatively, infection may trigger activation of an immune response specific for the oligodendroglia or the myelin components themselves. In the latter case, disease may follow an upper respiratory tract or other infection and primarily take the form of a demyelinating process. This disease is commonly termed postinfectious encephalomyelitis or acute disseminated encephalomyelitis.
Cause
The potential viral causes are multiple; however, enteroviruses, herpesviruses, and arboviruses are responsible for most disease (see Table 95-1). Enteroviruses account for up to 99% of cases of aseptic meningitis when a cause is identified.127 Enterovirus meningitis in older children and adults is typically self-limited and associated with few complications. In contrast, enteroviral infections in neonates may mimic bacterial sepsis, and CNS involvement is often manifested as encephalitis. Parechovirus, another cause of meningoencephalitis in neonates, is a close relative of enteroviruses and clinically very similar.128
HSV is a common cause of CNS infection in individuals of all ages. During the neonatal period, HSV type 2, and to a lesser extent type 1, cause encephalitis because of the vertical transmission of the virus.129 In contrast, in older children and adults, most HSV encephalitis is caused by type 1. HSV-2, however, can cause benign aseptic meningitis in association with primary and recurrent genital infections.130 Other members of the herpesvirus family (CMV, EBV, VZV, and HHV-6) can also cause aseptic meningitis and encephalitis, albeit less commonly. CMV encephalitis occurs mostly in immunosuppressed individuals but may occasionally appear in otherwise healthy individuals.131,132 EBV aseptic meningitis and encephalitis present with or without the classic findings of infectious mononucleosis.133 Acute cerebellar ataxia is a common and usually benign complication of chickenpox. VZV encephalitis can sometimes occur in immunocompetent individuals,134,135 but more frequently occurs in immunocompromised individuals following days, weeks, or months after a case of varicella or zoster. Zoster encephalitis can be complicated by small- or large-vessel vasculitis (granulomatous arteritis), which carries the potentially serious consequences of infarction.134,136 HHV-6 has only rarely been reported to cause encephalitis in healthy children during primary infection,137 whereas it appears to be a more common problem for immunosuppressed patients, such as those receiving stem cell transplants.138,139
Arboviruses (arthropod-spread viruses) are important causes of aseptic meningitis and encephalitis. The specific arbovirus determines the epidemiology, morbidity, and risk of death of associated disease. The La Crosse and St. Louis encephalitis viruses account for most arboviral CNS infections in the United States. The La Crosse virus is found mainly in the Midwest, typically occurs in the summer and early fall, and is associated with a relatively low death rate. The St. Louis encephalitis virus occurs in every state but is more common in the Midwest, Florida, and Texas and has been responsible for large urban outbreaks.140,141 Eastern equine virus occurs less frequently, and mainly in the Northeast and Southeast, but carries a high rate of morbidity (70% to 80%) and death (20% to 80%).142,143 West Nile virus encephalitis first appeared in the United States in the summer of 1999 in New York state.144 Over the following summers, the West Nile virus moved southward and westward across the United States, infecting both animals and humans. Most individuals infected with the West Nile virus are symptom free or experience flulike illness; however, older individuals and those with underlying immune deficiency can experience encephalitis that may result in death. In addition to the more typical presentation of encephalitis, an acute flaccid paralysis has also been associated with West Nile virus infection.145
A number of viruses are infrequent causes of encephalitis, including mumps, influenza, and lymphocytic choriomeningitis viruses (LCMVs). Historically, mumps virus accounted for a large proportion of aseptic meningitis and encephalitis cases in the United States.146 Currently, because of the widespread use of the trivalent MMR vaccine, meningitis and encephalitis due to mumps are extremely rare.147 Influenza has been associated with encephalitis/encephalopathy, especially in Japan. In a national survey representing the 1998-1999 season, 142 cases, most occurring in children younger than 5 years, are reported.148 LCMV is an infrequently recognized cause of meningoencephalitis. This virus is found in the urine, droppings, and saliva of infected mice, guinea pigs, and hamsters, and disease in humans arises after exposure to these substances.
Postinfectious encephalomyelitis refers to an acute self-limited demyelinating process most commonly following viral respiratory infections and varicella. In contrast, subacute sclerosing panencephalitis (SSPE) and progressive multifocal leukoencephalopathy (PML) are two chronic, usually fatal, demyelinating diseases due to measles and JC virus, respectively. SSPE most commonly follows 5 to 10 years after natural measles infection. SSPE is extremely rare in the United States; however, it may occur as often as one case per a population of 10,000 in areas of the world where the MMR vaccine is not widely used.149 PML is also rare, usually affecting those with AIDS or, rarely, those with other serious immunodeficiencies. Transverse myelitis has been most frequently associated with enteroviruses; however, VZV,150,151 CMV, influenza A,152 and hepatitis A153 have been reported causes, even in immunologically normal individuals.
Clinical Presentation
Historic clues and physical findings can be helpful in focusing the search for an etiologic agent. Travel or residence in areas where arboviruses are endemic during the appropriate season for arthropod transmission (typically summer months) and a history or evidence of insect bites should raise suspicion for arboviruses. Seasonality also plays a role in enteroviral diseases, because in temperate climates enteroviruses are more prevalent during summer and fall months. History of a mother with recent symptoms consistent with viral illness (fever, sore throat, gastroenteritis, rash) should raise suspicion of enterovirus in a neonate with encephalitis or sepsis-like illness. VZV encephalitis and myelitis typically follow chickenpox or zoster by weeks to months and commonly occur in older individuals or those with immunosuppression, such as transplant recipients.154 VZV encephalitis may be complicated by CNS vasculopathy and resulting infarctions. Chronic encephalitis/meningitis due to enteroviruses occurs in individuals with agammaglobulinemia. Chronic or relapsing encephalitis may also be due to VZV, measles (SSPE), or rubella (progressive rubella panencephalitis), though the latter two are extremely rare with the current widespread use of the MMR vaccine. HIV itself may cause encephalopathy/encephalitis or may also be associated with certain opportunistic infections such as PML. Significant exposure to rodent droppings should raise concern for LCMV. Finally, history of exposure to a bat should raise the concern for rabies.
Cerebrospinal fluid (CSF) findings in aseptic meningitis typically include a normal glucose level, a normal to slightly elevated protein level, and a pleocytosis of up to 1000 cells/μL. The pleocytosis is classically monocytic (>80%); however, there can be an initial predominance of polymorphonuclear cells in the first 48 hours of illness.155 CSF findings in encephalitis can be normal, or there may be pleocytosis and elevated protein levels. The results of brain computed tomography (CT) and MRI studies are usually normal in viral meningitis, whereas disease is often seen in the setting of viral encephalitides. In general, CT scan is relatively insensitive for detecting acute encephalitis. MRI is the more sensitive study for detecting disease because of its ability to detect altered water content (see Chapter 56).156 In acute viral encephalitis, early findings include edema with minimal contrast enhancement. As disease progresses, edema and enhancement become more obvious and may be accompanied by mass effect, hemorrhagic changes, and necrosis. As the inflammation resolves, atrophy may become prominent. In HSV, imaging studies may reveal edema and enhancement, often first involving the temporal lobes with subsequent spread to other areas of the brain. Changes can ultimately progress to atrophy, multicystic encephalomalacia, and gyriform high attenuation, especially in children.157,158 In postinfectious encephalomyelitis, the lesions may be seen throughout the CNS. The lesions are more readily elucidated by MRI and primarily involve the white matter, although gray matter may also be involved.
Exotic Viral Diseases
With both the increase in foreign travel and the threat of bioterrorism, the potential to treat a child with an exotic viral disease exists. Although discussion of these infections, which include Andes virus, B virus, monkeypox, and the hemorrhagic fever viruses (Ebola virus, Marburg virus, Lassa virus, Crimean-Congo hemorrhagic fever virus, Argentine hemorrhagic fever virus, Bolivian hemorrhagic fever virus) is beyond the scope of this chapter, these infections should be kept in mind. If one of these agents is suspected, then the patient and patient garments should be contained in a single room, and infection control, infectious disease specialists, or the public health department should be called immediately.159–161
Diagnosing Viral Disease
If a viral cause is suspected, a few diagnostic studies can be performed immediately. Acute-phase serum should be held for later interpretation. It is critical that this specimen is drawn before administration of IVIG or blood products. Samples for viral cultures and PCR testing should be collected from the appropriate sites with Dacron or rayon swabs with plastic shafts (or other specific swabs appropriate for testing by PCR). Both cotton and wood inhibit viral growth and may contain substances that inhibit the enzymes used in PCR. The virology laboratory should be informed of the diagnosis or suspected pathogens because the cell lines chosen for inoculation vary by what virus is suspected. Nasal washes/swabs and swabs of the base of a vesicle or ulcer (for VZV, HSV) should include good cellular content, because fluorescent antibody assays stain cells and the more cells available, the more sensitive the assay. Table 95-2 outlines appropriate samples and testing for a number of specific viral pathogens.
Table 95–2 Potential Diagnostic Tests and Corresponding Specimens for Diagnosis of Viral Pathogens
| Viral Agent | Specimen | Recommended Diagnostic Tests∗ |
|---|---|---|
| Adenovirus | < div class='tao-gold-member'> Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|