(2)
Icahn School of Medicine at Mount Sinai, New York, NY, USA
It is Monday morning, warm even at this early hour. By midday it will be a scorcher. I hope the air conditioner in the OR is working, but you never know. I was given a brief description of this OR by a colleague who had worked there; “they have everything you need… you will be fine.” The space is cramped with lots of equipment, some of it older than I am. I look for the capnograph on the monitor and realize it appears to be missing one. The anesthesia machine is a Drager Narkomed®; there is a circuit, a bag, and an isoflurane vaporizer but no ventilator. It is like a dinosaur but without a heart. I rifle through the drawers of the cart and scavenge a few items so I can set up for a case. A woman I have never seen before hands me a liter of normal saline and asks if there is anything else I will need, “and the first patient is waiting to see you,” she says. Who among us has not experienced the stress and excitement of working in a new environment?
The OR described above is a back room of a fancy Park Avenue plastic surgery office in Manhattan. The sentiment described above is only a fraction of the intensity of emotions experienced when one is on a mission in a foreign setting. As anesthesiologists we endeavor to control our environment and maintain homeostasis in our patient. Yet by definition, we face the unknown every day.
When I recruit and prepare anesthesia attendings and residents for international missions, I encounter the same cycle of emotions and anxiety. The most honest advice I can offer to new volunteers is this: “No matter how well you prepare for this mission, you will be surprised and challenged in ways you cannot imagine. No matter how hard you feel you have worked on any given day, you will work harder while on a mission… and you will love every minute.”
But back to the mission: You arrive to your destination and explore your new work environment. The immediate task is to prepare the available tools and equipment for any eventuality that may present itself. In both examples of new environments, the ORs on Park Avenue and in the lower and middle income countries (LMIC), the anesthesiologist feels isolated. Alone he/she has to set up for a safe and successful anesthetic with the resources at hand. In a sense, all small surgical groups travelling abroad undergo a learning process, improving efficiency with the acquisition of experience.
While leading the anesthesia team during a university medical school surgical trip organized by second and fourth year medical students, I was required to assign each of the three available operating rooms to our anesthesiologists. Each room had its advantages and disadvantages. One was small and cramped, without windows for the scavenging tube. Another was large and spacious but had three individual monitors for electrocardiography, pulse oximetry, and blood pressure. In an effort to be fair, we planned to rotate anesthesiologists through the rooms. Toward the end of the week, we realized that fairness was not as important as “owning the room.” Each rotation meant that the anesthesiologist had to ascertain the nuances of a new space. On subsequent missions to the same location we planned for each anesthesiologist to stay in his/her own room for the duration of the trip. Once the room was set up with equipment as desired, anesthesiologists were able to stock the drawers and shelves, as they preferred, creating a familiar work environment.
We decided that on each surgical trip we would create a guide or “playbook” in order to record vital practical information about the experience. It included lists of equipment and drugs carried from the USA, identifying surplus and what was lacking. It contained information about personnel at the site, contact information, and assistance provided. We described rooms, equipment and workflow, so that good processes could be repeated and others improved. This guide provided a vertical transition of information from year to year. Each group learned from the experience of their predecessors.
An early group realized that local staff members, after having worked with our team for a few days, brought in patients who were friends or relatives for procedures. They were not screened on our first day and we did not recognize them. We were told that we had screened them and must have lost the records. It was a compliment to our work that the staff was bringing us patients, but suddenly accommodating an additional patient was disruptive to our workflow and planning. The next year our team secured an ID band on each patient assigned to have surgery as the final step in the screening process. Patients were told not to remove the wristband until after they were discharged from our care.
Another group complained that in order to contact our team on the wards, they had to ask the head nurse in the OR suite to notify the ward nurse who may or may not have time to find someone. On subsequent trips we brought walkie-talkie sets which improved our communication efficiency.
Our approach to surgical trips was developing and maturing over time, but the evolution was slow. It would take a year to implement a new idea and see the result. Our “playbook” information was 11 months old. Even if were returning to the same site, many changes may have occurred. Changes such as new building, a new OR, recently donated equipment from another group or a construction project which might disrupt our power supply could occur during our absence. Communication with our hosts by phone or email was poor. At best we managed to set a date of arrival, but not much information was shared about the changing conditions.
We knew that other groups visited the same site throughout the year. There was a schedule on the wall of the OR office. We recognized some names of organizations listed. Had there been a forum to share information about a particular location among the volunteer caregivers, our information could be “horizontal.” We could potentially learn from others who used the facility and provided care the week before our arrival. We could be updated with the latest information. If they wrote a “playbook” of their mission and shared it, we could be much more effective. We could implement strategies they had contemplated and report back to them and the group following us for the benefit of all.
To date, there exists no such forum. Journal articles appear with increasing frequency. Greater organization of effort and research of surgical missions is becoming a focus of many medical institutions [1]. Guidelines for success have been proposed [2]. Global health institutes are popping up and are endeavoring to standardize the approach to travel medicine or “medical tourism.”
References
1.
Fisher QA, Fisher G. The case for collaboration among humanitarian surgical programs in low resource countries. Anesth Analg. 2014;118(2):448–53. PMID: 24445642.
2.
Grimes CE, Maraka J, Kingsnorth AN, Darko R, Samkange CA, Lane RH. Guidelines for surgeons on establishing projects in low-income countries. World J Surg. 2013;37(6):1203–7. PMID: 23474858.
Letter 2: Challenges in Delivering Care to a Pediatric Patient with a Difficult Airway in a Resource-Poor Setting Grace Hsu
Grace Hsu3
(3)
Department of Anesthesiology and Critical Care Medicine, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA
Located in the horn of Africa, Ethiopia is home to 92 million people. It is one of the world’s poorest countries, with a per capita GDP of $410. The Black Lion hospital in the capital, Addis Ababa, is one of Ethiopia’s major referral hospitals. Of the 1,421 physicians in the country, 106 are surgeons and 14 are anesthesiologists [1]. Nurse anesthetists administer anesthesia for the rest of the country. Access to surgical care is poor—thus limiting most surgery to emergent or late presentations.
It was my third day in Ethiopia. I was at the Black Lion Hospital in Addis Ababa to teach students in the Master’s of Science in Nurse Anesthesia program. I arrived at the hospital and crowded around the operating schedule board at 7:00 am with a large group of other hospital staff to see the assignments for the day. I was to cover two rooms, a pediatric and a urology room. The first pediatric case was removal of a cervical lymphangioma in a 7-month-old boy.
With one of the students I went to the preoperative holding area to see the pediatric patient. A very large mass distorted the boy’s right jaw, face, and neck. His face, lips, and neck were swollen to a point beyond recognition. The parents gave us the history: The baby was born with a right neck mass but was otherwise healthy. At around 2 months of age, the mass began increasing in size. By 5 months, he had extreme difficulty eating and was failing to thrive. He drooled constantly, and was unable to swallow saliva effectively. One week prior, he came to the hospital for the first time in his life, with pneumonia. He was in respiratory distress and required oxygen 2 l/min to maintain adequate saturation. The pediatric surgeon recommended surgery immediately as development of complete airway obstruction was imminent.
On exam, the infant was thin and listless. The mass and swelling had stented his mouth open to its maximum, but with little patent area in his oropharynx. He was tachypneic and had decreased breath sounds in the right upper lung fields. A CT scan film indicated that the mass compressed his upper trachea and appeared to extend beyond the thoracic inlet and into the anterior mediastinum. Portions of his right lung were whited-out from pneumonia.
I expressed concern about a difficult airway to the pediatric surgeon, given the severe degree of swelling. We expected difficulty ventilating, especially with positive pressure, given the small opening in the oropharynx and the potential for the mass encroaching into the anterior mediastinum. Even if we were successful in passing an endotracheal tube through the patient’s vocal cords, we might not be able to pass beyond the tracheal narrowing. The surgeon felt she would not be able to provide an emergency surgical airway, given the neck distortion and high likelihood of numerous collateral vessels in the region. The only airway adjuncts in the hospital were supraglottic airways, an adult-sized gum elastic bougie and an adult bronchoscope.
I asked a Norwegian anesthesiologist who was there teaching residents for 6 weeks, and another recent graduate of the Ethiopian residency program at the Black Lion Hospital to be present for induction. I outlined a plan for a group of students: start with an inhalation induction to preserve spontaneous ventilation and allow the anesthesia student two attempts to secure the airway. If she was unsuccessful, an anesthesiologist would then take over. Our only size-appropriate airway adjunct was a supraglottic airway. We planned to use a small 3.0 cm cuffed endotracheal tube in anticipation of tracheal narrowing.
Induction and intubation proved difficult. The nurse anesthesia student induced anesthesia with halothane via mask. With loss of muscle tone in the oropharynx, the patient began to obstruct and oxygen saturation dropped rapidly. Despite earlier discussion of the plan, under stress, the student began to apply positive pressure. Although ventilation was not adequate, it was sufficient to keep the patient unconscious with inhalational anesthesia but also weak enough to obstruct. The nurse (anesthesia student) attempted to intubate the patient twice—but was unable to see the larynx. Next, the Ethiopian anesthesiologist attempted direct laryngoscopy. With a shoulder roll and laryngeal pressure, she obtained a grade III view and placed a 3.0 endotracheal tube successfully through the narrowed portion of the trachea. Although there was no air leak around the deflated cuff, we decided to continue the case with this tube. We gave a single dose of dexamethasone to minimize swelling (Figs. 28.1 and 28.2).
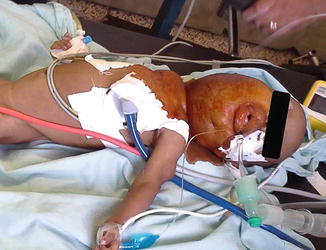
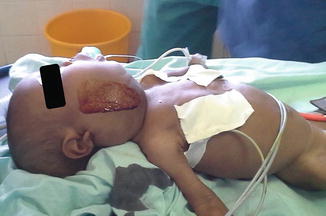

Fig. 28.1
The cervical lymphangioma made both mask ventilation and laryngoscopy difficult in a setting with limited airway adjuncts (supine view)

Fig. 28.2
Lymphangioma lateral view
The surgical portion of the case went smoothly, other than significant blood loss from the venous collaterals draining the head and neck. A full blood volume was replaced. While considering postoperative care, I learned that the hospital did not have a pediatric-sized ventilator. The 6-bed intensive care unit had two adult ventilators that would likely cause volutrauma if used for a child. Limited medical and nursing staff overnight meant that no one would be available to manually support the baby’s ventilation. Thus, the patient would need to breathe spontaneously through the small endotracheal tube without any support until extubated. I again enlisted the help of the Norwegian and Ethiopian anesthesiologists. The Ethiopian anesthesiologist explained that it was extremely rare for a pediatric patient to be intubated and ventilated in the hospital. We also discussed the possibility that the patient would weaken throughout the night, breathing through a long, narrow tube. Acute airway obstruction the night after surgery was also a significant risk [2]. We decided to extubate the child—again, preparing for a difficult reintubation. The 30 min that followed proved that the patient could not tolerate extubation—the swelling from surgical manipulation was too great. The patient did not appear to have recurrent laryngeal nerve deficits as he was phonating; however, he quickly obstructed. We reintubated him—again, a difficult task.
Transfer to the intensive care unit itself was a feat. There were no fully functioning bag-valve masks with tubing to attach to an oxygen source. We improvised with intravenous fluid tubing to connect the bag-valve mask to an oxygen tank. After traveling 20 ft down the hallway in a metal crib with a large oxygen tank lying at the foot, one wheel of the crib broke off and nearly caused the crib and patient to topple. The elevator ride from the fourth floor operating rooms to the sixth floor intensive care unit was also precarious. (The rubber mat on the floor of the elevator car was ineffectively covering a hole one-foot in diameter).
The baby did well and was extubated by the ICU team on post-op day 1 and discharged home on post-op day 6.
The key learning points of this case were the following:
The history and physical exam are key for the anesthesiologist in formulating a plan and in determining the most likely things that will harm a patient perioperatively. In this case the three major considerations were a difficult airway, intraoperative hemorrhage and postoperative ventilation.
In resource-poor settings, where airway adjuncts are extremely limited, human resources may be just as important in a difficult situation. In this circumstance, the only airway adjunct appropriate for this 7 kg patient was a supraglottic mask airway. It is important to involve other airway experts and team members in the case early and discuss a plan.
Especially when there are language and cultural barriers, it is important to have a clear plan that all team members understand. In this case, it would have been prudent to not attempt mask ventilation after obstruction during induction. One should allow the patient to emerge from general anesthesia.
Given that this patient required this surgery for survival, there seemed to be little choice in performing the operation. Some might argue that such high-risk cases should not be undertaken because a bad outcome may jeopardize future missions. However, this decision falls more to the surgeon than to the anesthetic team. Consideration of postoperative resources and availability of adequate support is crucial to the decision.
References
1.
Chao TE, Burdic M, Ganjawalla K, Derbew M, Keshian C, Meara J, McQueen K. Survey of surgery and anesthesia infrastructure in ethiopia. World J Surg. 2012;36:2545–53.
2.
Ameh EA, Nmadu PT. Cervical cystic hygroma: pre-, intra-, and post-operative morbidity and mortality in Zaria, Nigeria. Pediatr Surg Int. 2001;17:342–3.
Letter 3: Great Planning: But Not Everything Works Ram Roth
Ram Roth4
(4)
Department of Anesthesiology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
For many years, anesthesiologists have embarked with surgical teams from Mount Sinai Medical Center in New York on short-term missions to underserved countries. Not only have we been able to help hundreds of people in these countries but we have also garnered considerable satisfaction and experience for ourselves. The stories of successful missions abound. On one recent mission, however, the outcome did not go according to plan and the results were disastrous.
Background
Perioperative care for patients at a mission site is in many ways similar to that provided in the USA with some adjustments or local availability of resources. A preoperative evaluation of all potential patients allows the surgical team to be able to prioritize candidates for surgery. Local physicians make the initial evaluation and selection. The teams of surgeons and anesthesiologists then have the opportunity to evaluate the need for surgery, cardiac risk indices, comorbidities, medications, and airway management considerations.
Standard preoperative evaluation is based on the guidelines of the mission site, the external organization involved, and/or hospital of mission origin. The mission site we visited had more stringent rules than apply at our institution in New York regarding preoperative evaluation, and requires cardiac clearance on all patients over the age of 40. This is possibly due to a shorter life expectancy and generally less prophylactic care received by potential surgical candidates. Alternatively, it may be included to ensure that all visiting surgical groups are satisfied with their screening process. Patients are usually “cleared for surgery” by local cardiologists before they come to the center. All patients are admitted the night before surgery in order to minimize transportation problems and ensure preoperative fasting. Anesthesia screening also includes assessment of the availability of appropriate support following surgery and determination that the patient will recover within the time frame of the mission with sufficient support mechanisms in place.
Case
A 60-year-old woman presented with a long-standing history of severe gastroesophageal reflux disease and upper abdominal pain. An open cholecystectomy was scheduled after the finding of multiple gallstones. She had a history of diabetes and hypertension. She repeatedly denied any cardiac symptoms, despite questioning by several health care workers at different levels. Medication included metformin, propranolol, enalapril, and glyburide, all of which she took regularly. She reported that her diabetes was well controlled. She had undergone two cesarean sections in the distant past under spinal anesthesia. She did not smoke or drink. There was no history of allergies.
On physical examination she was found to weigh 100 kg with a height of 161 cm (BMI 39). While not taking her medication her blood pressure was 144/91 and heart rate 78 and regular. No murmurs were identified and her lungs were clear to auscultation. A cardiologist had seen her in the recent past and she reported that all was well. The plan was to schedule her operation later in the week.
On the penultimate surgical day of our mission, 3 days before our departure, the patient was transported to the operating room at 16:40 after having fasted for 23 h. Standard monitors were applied and an intravenous infusion started. Induction and intubation proceeded uneventfully. Medications included fentanyl 100 ugm, propofol 200 mg, morphine 8 mg, rocuronium 20 mg. Anesthesia was maintained with sevoflurane in oxygen. Vital signs remained stable for about 30 min into the operation (BP 110/70, heart rate 80). Thereafter, oxygen saturation fell to the 80 s and the blood pressure was unobtainable. Phenylephrine, epinephrine and atropine were administered. As the surgeon exposed the gallbladder, he noted that the liver appeared congested (Fig. 28.3). After 10 min the vital signs stabilized. Ventilation continued with oxygen 100 % while the gallbladder was exposed and resected. Subsequently, blood pressure was unobtainable and she was resuscitated with crystalloid and vasoactive agents. The pulse returned and the surgery proceeded with only 5 min remaining in the case. The patient continued to require repeated boluses of phenylephrine and vasopressin for episodes of hypotension. Attempts to gain additional intravenous access were not successful. She was given a total of 1 liter of crystalloid and was breathing spontaneously via the endotracheal tube as the bandages were applied. However, she remained unstable; blood pressure continued to decrease despite vasopressor therapy and additional crystalloid via a groin central line. Pulseless electrical activity developed and full ACLS protocol was instituted in the cramped operating room. She regained her pulse on several occasions but the rhythm could not be sustained. After more than 1 h of cardio pulmonary resuscitation she was pronounced dead.
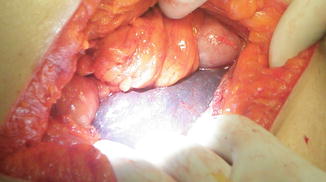

Fig. 28.3
Congested liver
Discussion
An event such as this is devastating, not just to the family but also to the entire team of health care providers, both visitors and local. We held debriefing sessions with the local doctors that night and with the operating room staff the next day.
After returning to our guesthouse, the entire mission team participated in an emotional debriefing. Members of the team described how the experience affected them. Medical students and attendings openly cried, the former for having experienced their first death in the operating room and the latter for having experienced one so unexpected.
The next day was originally planned to be the last day of surgery. The team felt that doing the few small cases scheduled would be inappropriate and those cases were cancelled to be performed on a subsequent mission. We began the day with a debriefing for the nursing and operating room staff. Educationally, they were most curious about our teamwork during cardiopulmonary resuscitation. They had never seen a “code” except on TV shows. We explained how we all undergo ACLS training and practice responding and appreciated that this was an area of focus for future missions. This certainly left us with the idea that our next mission would include an program on cardiopulmonary resuscitation for the staff.
We also explained that in our practice we investigate every detail of any death or major complication. We arranged for a full review of all past records and spoke with the family. Some relevant pages from her prior cardiac studies had been removed and were determined to be “lost.” Eventually, the local director of anesthesiology learned that the patient had been diagnosed with severe heart disease, which had been revealed to previous visiting teams and to local surgeons. Surgery had been ruled out in the past. Since her colic symptoms were intensifying, she determined, in consultation with her family, not to admit to any heart problems to our team. She was rehearsed in screening questions and her previous answers that would not allow her surgery to proceed.
We were anxious to determine the pathology and suggested an autopsy in the public city hospital. The family refused for religious reasons. The hospital administrator then beneficently made arrangements to cover funeral expenses. Even though we could not determine the cause of this tragic outcome we worked to obtain all additional information. The remainder of the day was spent in obtaining as much information as we could and taking care of the patients in the ward and bonding with our friends and colleagues in the hospital.
Upon returning to our institution, we conducted a formal mortality review chaired by the Dean of Global Health. All members of the mission team were interviewed and the patient’s records as well as perioperative surgical and anesthetic charts were reviewed. We reexamined our protocols and concluded that little could be done to uncover information that was deliberately withheld. Students and residents were given another opportunity to discuss the emotional impact of the tragic event. A review panel added a cardiac risk assessment and a mini-stress test to the preoperative evaluations for all missions. The Global Health office reviewed the institute’s malpractice coverage to ensure that missions were protected for claims brought at home and in countries we visit.
Our next mission to the site is anticipated and we were assured that we would be welcomed to continue our work there.
Conclusion
Despite the best intentions and due to circumstances beyond our control, poor outcomes may still occur. That is the nature of medicine and surgery in the developing world. Cooperation with the host countries should be in place for success. There must exist some degree of trust in the local physicians and belief that patients will give accurate information, as most do. Patients must be evaluated carefully by skilled clinician and rejected if necessary when their physical findings or history is not compatible with obvious objective clues (e.g., morbid obesity). An understanding of attitudes and what may be considered reasonable and acceptable behavior in other countries is very hard for many, especially young health care workers from our modern institutions. Counselling should be made available to the team, recognizing the tragedy of the case at several levels and also acknowledging that global surgical missions are a beneficial endeavor.
Letter 4: Life-Saving “The Magic” Wire Miodrag Milenovic
Miodrag Milenovic5
(5)
Department of Anesthesiology and Intensive Care Medicine, Clinical Center of Serbia, Belgrade, Serbia
Difficult airway workshops are often highly attended and the most popular events at international congresses and regional meetings. Serbian anesthesiologists, just like others, are very keen to attend them and later on their return home to give a “hard time” to the hospital management asking them to buy all those hi-tech, often very expensive devices for difficult airway management. Should devices be obtained, not all of us are practiced to use them, because there is no official obligation to do so and it is time consuming.
There are a number of clinical situations when the anesthesiologist and intensivist cannot completely visualize entrance into the trachea. Very often this was the moment when we realized that not so many of us are skillful enough to properly use the bronchoscope or other techniques available to us.
One of the international scholars and an AAF alumnus returned from the Postgraduate Assembly in New York with a few pieces of long gum elastic bougies, or Eschmann Stylets. These are tracheal tube introducers, a flexible device that is 60 cm (24 in.) in length, and 15 French (5 mm diameter) with a small “hockey-stick” angle at the far end. It can be a useful adjunct for the persistent epiglottis-only-view situations. Although this product has been on the market for almost 30 years now, probably because of the small population in Serbia and the low cost, we have not been offered it by medical industry representatives in our country. It was not on the market in the region.
From that time 10 years ago, when the department was given several pieces, as a gift from the colleague who came back from abroad, long gum elastic bougies have became the necessary equipment for difficult airway management and for re-intubation of an already difficult intubated patient. My colleages (anesthesiologists and nurses) call it “the blue wire” or even “the magic stick,” because it has saved a number of lives over the last decade, especially before we became more efficient in bronchoscope use.
A case in point: A 66-year-old man had been hiding out in a tree for several hours, hoping to shoot a deer. He had drunk some beer and, feeling rather feverish, had ingested some cold remedy. He was soon fast asleep and fell to the ground, dislocating his neck at C7. In the hospital he was initially stabilized in a collar but over the next 24 h, his sensory level rose to C5. The decision was made to take him to the operating room for surgical stabilization. He had no history of prior surgery. A plan was made to perform rapid sequence induction as he had not eaten in 30 h with in-line stabilization. Immediately after induction, large amounts of fluid and undigested food were regurgitated. The larynx could not be visualized. Finally, with fiber-optic scope, the airway was secured. Surgery proceeded without incident. He appeared to be awakening, and was trying to remove the endotracheal tube. We placed a bougie through the tube prior to extubation. Some 4 h later, he experienced some respiratory distress and we were able to quickly secure the airway this time over the bougie. He was successfully extubated 30 h later.
Being a part of the moderately developed medical system but still less affluent world, there is no doubt that our hospitals need to renew medical equipment, but the professionals need to be more realistic, pragmatic, and skilful to be able to deal with difficulties, not relying only on the latest generation of hi-tech devices, because we may need some time to achieve much.
From the moment we become aware of such simple but excellent adjuncts as gum elastic bougies, it is part of our official demands and we still do not have suppliers of such simple and cheap airway adjuncts. It is still not on the market. So, a number of such easy-to-carry, inexpensive, and simple-to-use adjuncts should be necessary as disposable equipment for all outreach medical missions, because small things like gum elastic bougies can change anybody’s life.







