KEY POINTS
The subclavian approach is preferred for placement of central venous catheters (CVCs).
Real-time ultrasound may reduce the mechanical complications associated with CVC insertion.
Chlorhexidine-based skin antiseptic solutions reduce the incidence of catheter-related bloodstream infections as compared to povidone-iodine.
Almost 50% of hospital-acquired bloodstream infections are caused by staphylococcal species.
CVCs should not be replaced nor exchanged over a guide wire on a routine basis.
Central venous catheters (CVCs) have become an integral part of delivering care in the modern intensive care unit (ICU). In fact, the CDC estimates that in US ICUs there are 15 million CVC days per year (total number of days patients are exposed to CVCs).1 Indications for placement of CVCs include invasive hemodynamic monitoring, administration of vasoactive drugs, administration of caustic agents (eg, chemotherapy), administration of parental nutrition, renal replacement therapy, large bore venous access for rapid administration of fluids, and long-term venous access. This chapter will focus on the use of CVCs in the ICU setting. Thus, long-term tunneled catheters used for hemodialysis and peripherally inserted central catheters (PICC) will not be discussed.
PLACEMENT OF CENTRAL VENOUS CATHETERS
The clinical presentation often dictates the type of catheter to be inserted. For example, a patient with a hemodynamically significant gastrointestinal hemorrhage may only require a single lumen, large bore CVC for volume resuscitation in addition to a peripheral IV, whereas a neutropenic patient with septic shock may require a triple lumen CVC in order to simultaneously administer vasoactive drugs and antibiotics. Importantly, most evidence suggests that the number of catheter lumens does not affect the rate of CVC infectious complications.2,3 Once the type of catheter has been selected, an anatomic site for insertion needs to be determined. The optimal anatomical location for insertion of CVCs has been a matter of debate for many years. In 2001, Merrer and colleagues published a study of 289 patients who were randomized to have their CVCs inserted in either the femoral or subclavian vein.4 Patients with femoral vein catheters had a dramatically higher incidence of infectious complications (19.8% vs 4.5%; p < 0.001) as well as thrombotic complications (21.5% vs 1.9%; p < 0.001) as compared to patients with subclavian catheters. The overall sum of mechanical complications (arterial puncture, pneumothorax, hematoma or bleeding, air embolism) was similar between the two groups. To date, there are no randomized trials comparing subclavian versus internal jugular catheters with regard to infectious complications, though observational studies suggest a lower rate of infectious complications with subclavian catheters and a similar rate of mechanical complications.5,6 A recent Cochrane review on comparison of central venous access sites in 2007 did suggest that subclavian catheters had lower rates of colonization (defined as culture tip with >103 colony-forming units) and major infectious complications (ie, clinical sepsis with or without bacteremia) when compared to the femoral site.7 As a result of these and other8 studies, the CDC recommends that, if not contraindicated, the subclavian vein should be used for the insertion of nontunneled CVCs in adult patients in an effort to minimize infection risk.
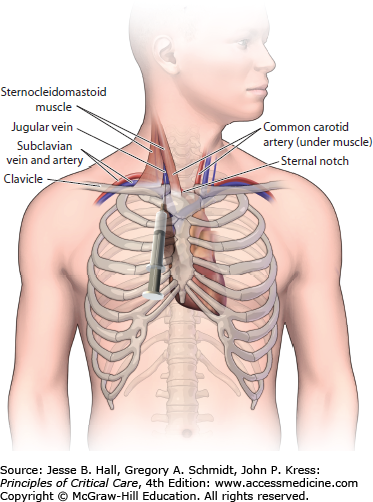
INFRACLAVICULAR SUBCLAVIAN APPROACH
Prior to the insertion of an infraclavicular subclavian CVC, a small rolled up towel should be placed between the shoulder blades to move the vascular structures more anterior. After the subclavian area has been sterilely prepped and draped (see below) and local anesthesia has been administered, the patient should be placed in Trendelenburg position. The arm should be positioned at the patient’s side so that the shoulder, clavicle, and sternal notch are aligned and perpendicular to the sternum. The subclavian vein arises from the axillary vein and travels beneath to the clavicle and inferior to the subclavian artery prior to joining the internal jugular vein and forming the brachiocephalic vein. Thus, the clavicle provides a good anatomic landmark for the insertion of a subclavian CVC. The skin should be entered with an 18-gauge introducer needle 1 to 2.5 cm below the inferior edge of the clavicle and 2 to 4 cm lateral to the midpoint of the clavicle. Once the needle is directly underneath the clavicle, it should be advanced toward the sternal notch making sure that the needle remains in the plane immediately below the clavicle. If no blood return is obtained, then the needle should be pulled back and directed more cephalad. Slight backpressure should be placed on the plunger of the syringe any time the needle is advanced or withdrawn so that blood return can be visualized when the vessel is cannulated. After the vessel has been accessed, the modified-Seldinger technique is utilized to complete insertion of the CVC. Inexperienced operators often have difficulty knowing when the needle is directly underneath the clavicle and are appropriately fearful of puncturing the visceral pleura. Thus, we often utilize a slightly different approach when supervising an inexperienced operator. Once the skin is entered, the operator is instructed to find the clavicle with the tip of the introducer needle. After the edge of the clavicle is reached, and more local anesthesia is given in the area, the introducer needle is retracted slightly (1 cm) and redirected in a more posterior direction by pushing down on the syringe and needle as a unit with the nondominant hand. This prevents the inexperienced operator from advancing the needle at a steep angle toward the underlying visceral pleura of the lung while attempting to locate the posterior border of the clavicle. Once the needle is “walked down” the bone and is positioned underneath the clavicle, it is then redirected toward the sternal notch and advanced slowly while applying backpressure to the syringe (Fig. 27-1).
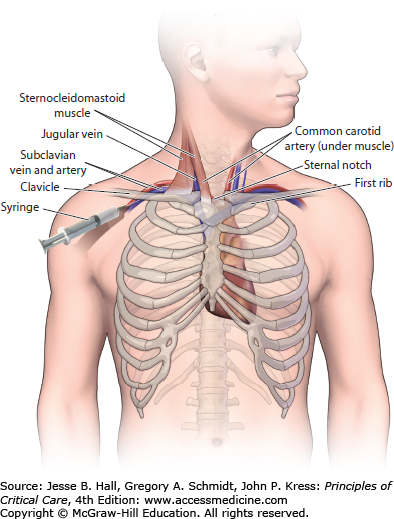
CENTRAL INTERNAL JUGULAR APPROACH
If the patient’s anatomy (scar from previous vascular access) or coagulation disorder prevents the use of the subclavian vein, then the internal jugular vein should be utilized for placement of a CVC. The central approach to placing an internal jugular catheter uses the triangle formed by the two heads of the sternocleidomastoid muscle and the medial portion of the clavicle as the anatomic landmark. Most commonly (though not always), the internal jugular vein is lateral to the carotid artery and both vessels run through the triangle beneath the sternocleidomastoid muscle. After the patient has been sterilely prepped/draped and local anesthesia has been administered, the patient is placed in Trendelenburg position and the head is rotated slightly toward the contralateral side such that the carotid artery can be palpated in the apex of the triangle. The nondominant hand is used to lightly palpate the carotid artery with careful attention not to place too much pressure on the skin as this can alter the position of the internal jugular vein. A smaller (eg, 21-gauge) “finder needle” is often used to locate the vessel prior to using the introducer needle (18-gauge). This “finder needle” should enter the skin at the apex of the triangle and be advanced at an angle of 60° above the plane of the skin. If the nondominant hand is able to delineate the course of the common carotid artery, then the needle should be advanced along a similar line just lateral to the carotid artery as both vessels are contained within the carotid sheath. If the carotid artery cannot be palpated with the nondominant hand, then the needle should enter the skin at the apex of the triangle at a 60° angle to the skin and be advanced in the direction of the ipsilateral nipple. If the needle is inserted to a depth of 3 cm without achieving good blood return, then the needle should be pulled back slowly while applying constant backpressure to the plunger of the syringe and redirected more medially before slowly advancing the needle again. After the vessel has been cannulated the modified-Seldinger technique is utilized to complete insertion of the CVC (Fig. 27-2).
POSTERIOR INTERNAL JUGULAR APPROACH
The posterior internal jugular approach is an alternative to the central internal jugular approach that may be used if there is concern that the patient would not be able to tolerate a procedure-related pneumothorax (high positive end-expiratory pressure and/or high FiO2 requirements). The puncture site is posterolateral to the sternocleidomastoid muscle, immediately cephalad to where the sternocleidomastoid is crossed by the external jugular vein. The needle should be directed beneath the muscle and advanced in an anterior and inferior direction toward the sternal notch. If blood return is not obtained, the needle should be pulled back and redirected slightly more posterior until venous blood is obtained. Because unintentional carotid artery puncture is more likely with this approach, a “finder” needle should be used prior to cannulation with a large bore needle.
ULTRASOUND-GUIDED PLACEMENT
Ultrasound guidance to assist in the placement of CVCs is a strategy with growing interest. Early studies did not suggest that ultrasound guidance for placement of CVCs improved outcomes. For example, Mansfield and colleagues reported that ultrasound guidance did not impact the rate of complications or failures in 821 patients randomized to standard insertion procedures with anatomic landmark guidance versus ultrasound guidance for subclavian vein catheterization.42 It is noteworthy that this study used ultrasonography to locate the subclavian vein, but did not use real-time ultrasound guidance for the actual venipuncture. In contrast, several more recent studies have compared the use of real-time ultrasound with the use anatomic landmarks during the insertion of both subclavian and internal jugular venous catheters. These studies showed decreased failure rates, decreased complications, and an increased rate of successful catheter placement on the first attempt with use of real-time ultrasound.43,44 A recent review of CVC complications reported a 6% to 10% incidence of mechanical complications with the insertion of subclavian and internal jugular CVCs.6 Given the frequent use of CVCs in the ICU and the risk of mechanical complications, it seems prudent to utilize real-time ultrasound during the insertion of CVCs if it is available, especially for patients with coagulation disturbances or unclear anatomical landmarks. As a result, the British National Institute for Clinical Excellence (NICE) and American College of Surgeons have recently recommended the uniform use of ultrasound guided catheter placement for elective if not all central access catheters in their respective guidelines.45,46
INFECTIOUS COMPLICATIONS OF CENTRAL VENOUS CATHETERS
Catheter-related infections (bloodstream infection, catheter colonization, or an exit-site infection) are thought to arise via several different mechanisms: Skin flora from the insertion site can migrate down the external surface of the catheter; the catheter hub can become infected with repeated manipulation; or hematogenous seeding of the catheter tip can result from a distant source of bacteremia.33 CVC-related infections are the most common cause of nosocomial bacteremia in critically ill patients.15 The incidence of hospital acquired, CVC-associated bloodstream infections (BSI) is collected by the CDC’s National Nosocomial Infection Surveillance System (NNIS) and is expressed as the number of BSI per 1000 CVC days. From 1992 to 2004 the rate of CVC-related BSI in adult ICUs ranged from 2.7 to 5.0 per 1000 catheter days.16 Diagnosis of a CVC-related BSI requires clinical symptoms of bacteremia (fever >38°C, chills, or hypotension) without another apparent source, and isolation of an organism from a peripheral blood culture with either a semiquantitative or quantitative culture of a catheter segment that yields the same organism and antibiotic sensitivities as the organism cultured from blood. In the semiquantitative culture method the catheter segment is rolled on a culture plate and considered positive if there are greater than 15 colony-forming units (CFU) of an organism. In the quantitative method the catheter is processed in broth and sonicated, followed by plating the broth on a culture plate. A positive culture requires growth of greater than 103 CFU.18 CVC-related BSI should be distinguished from catheter colonization, which only requires a positive semiquantitative or quantitative culture from a catheter segment. In addition to BSI and catheter colonization, a CVC can develop an exit-site infection defined as erythema, tenderness, induration, or purulence within 2 cm of the catheter exit site.18
The majority of pathogens causing CVC-related BSI are skin flora, which suggests migration of bacteria down the catheter as the mechanism of infection. This notion is supported by a study of pulmonary artery catheter (PAC) infections. This study of 297 PACs found that 80% of infected catheters showed concordance with organisms cultured from the skin at the insertion site.5 According to NNIS data from 1992 to 2004, slightly more than 50% of hospital-acquired BSIs were caused by staphylococcal species. The most common organisms isolated were coagulase-negative staphylococci (31%), Staphylococcus aureus (20%), Enterococcus (9%), gram-negative rods (14%), and Candida species (8%).16 There is also increasing resistance of the isolates— specifically, methicillin-resistant Staphylococcus aureus(59.5%), vancomycin-resistant Enterococcus (28.5%), and third-generation cephalosporin-resistant Klebsiella pneumoniae (20.6%).16 Although these resistance patterns were isolated from the ICU population, they were not risk adjusted or controlled by individual hospital resistance rates. Therefore, specific institutional resistance patterns must be considered when evaluating resistant bacterial infections. Given the frequency and cost associated with the treatment of catheter-related infections, there has been a great deal of research into reducing the rate of these infections.
Several interventions, implemented at the time of catheter insertion, have been shown to reduce the rate of catheter-associated infections. Implementation of an evidence-based practice for prevention of catheter-associated infections has been shown to be effective when implemented across multiple institutions. The Keystone ICU project enlisted over a hundred ICUs in Michigan to monitor and report the number of catheter-associated infections. Clinicians were educated about the evidence-based practice (ie, hand washing, using full- barrier precautions, cutaneous antisepsis using chlorhexidine, avoiding the femoral site when possible, and removing unnecessary catheters), provided with central-line carts, and completed checklists to ensure adherence to infection control practices.21 At 18 months of follow-up the mean rate of infection per 1000 catheter days decreased from 7.7 to 1.4. A subsequent study evaluated the sustainability of this quality improvement project and demonstrated that the mean rate of catheter-related infections remained low at 1.1 per 1000 catheter days with the ongoing implementation of this evidence-based algorithm.22 In addition to institutional quality improvement projects, simulation training not only improves competence in placement of CVC insertion, but also has implications for reducing the rate of catheter-associated infections. A recent study demonstrated that simulation training is superior to traditional apprenticeship model or video training alone when assessing sterile technique.23 Interestingly, simulation-based training was also associated with fewer catheter-related infections when compared to the traditional apprenticeship model (1.0 vs 3.4 per 1000 catheter days) and others.23,24 Thus, implementing evidence-based guidelines through quality improvement projects and simulation-based training are effective and sustainable methods in the prevention of catheter-related infections.

Full access? Get Clinical Tree