CHAPTER 67
Intrathecal Drug Delivery Trialing Procedures
INTRODUCTION
• Intrathecal drug delivery system (IDDS) trials offer the clinician an ability to utilize neuraxial medications in the patient and assess whether or not the patient has a positive response to the medications.
• By delivering substances in this format, prior to committing the individual patient to surgical implantation of the device, the physician can be cost-effective, selective, and most importantly improve patient efficacy and safety.
• There are 2 major categories of patients who would be considered for intrathecal pump infusion system trialing in the field of interventional pain management.
• The first group suffers from neuropathic, nociceptive, or mixed pain syndromes which may be related to cancer pain or noncancer pain syndromes. Trialing often involves a primary opioid, ziconotide, or a combination of medications to treat different types of pain.
• The second group suffers from poorly controlled spasticity, and involves the injection of baclofen.
• The mechanics of needle and catheter placement may be similar between these groups; however, the assessment of the individual patient, with respect to outcome, differs significantly.
• Trialing in the neuroaxis also varies based on the targeted area of drug delivery.
• Options include single shot injections or catheter placement with intermittent bolusing or continuous infusion, and the anatomical location may be the epidural space or the cerebral spinal fluid.
RELATIVE ANATOMY
Intrathecal pump infusion system trials are generally performed with placement of the needle below the conus, and the catheter in the area of the pain generator. The needle placement, generally, is performed between L1 and S1 in the interlaminar space. The specific anatomic points of consideration are as follows:
• Visualization of the thoracolumbar junction at the lowest rib level
• Visualization of the pedicles and spinous processes
• Identification of the interlaminar spaces
Other relative anatomy that should be considered includes:
• The body habitus of the patient
• The relative amount of lumbar lordosis present in the patient’s position on the table
• The orientation of rotation of the spine in a patient with scoliosis
• The integrity of the skin
• The paraspinal musculature
• The spinal ligamentous structures
• The ligamentum flavum and the dura mater
BASIC CONCERNS AND CONSIDERATIONS
There are some general concerns and considerations that would be applicable to all forms of IDDS trialing, whether the trial is performed as a single-shot, continuous epidural infusion or continuous intrathecal infusion.
The choice of interlaminar space:
• The location should be based not only on adequate visualization of the interlaminar space, along with adequate space visualized to accommodate needle placement, but should also be based on entering the spine at a level with good skin integrity.
• This includes an area absent of any evidence of skin breakdown or infection, and at a level where previous spine surgery has not been performed.
• Areas of previous surgery may have atypical spinal anatomy, and may lead to complications.
Immunocompromised patients:
• Patients with immunocompromised status may require preprocedure consultation and special antibiotic or preoperative care.
• They should be monitored very carefully for the development of infection, although this is a rare complication in short-term trials.
• Significant compromise of immune response may be an absolute contraindication to the procedure, and consultation with a infectious disease is warranted with white blood counts less than 2000.
• This may be of concern in those undergoing chemotherapy, suffering from malignancy, with advanced age, and with other advanced diseases.
Fluoroscopy:
• Radiolucent hardware, soft tissue masses, or other obstructions to needle placement may not be visualized on fluoroscopic imaging.
• Vigilance should be used in those cases where needle placement is difficult. Imaging may be enhanced with contrast in cases where there is uncertainty about location.
Other concerns:
• Patients with bleeding disorders may not be candidates for the procedure.
• Patients with thrombocytopenia that have platelet counts less than 50,000 may be at high risk for bleeding.
• Care should be administered in those with counts below 100,000 or with a downward trend.
• Platelet function is equally worrisome, and should be assessed by a hematologist if questions persist.
Difficult patient position:
• Patients with abdominal wounds or masses may have difficulty with lying in the prone position.
• Most trials can be performed with the patient in the lateral decubitus position which can be tolerated with or without the addition of sedation in most cases.
Absolute contraindications to the procedure include:
• Active spinal infection
• Localized infection at the area of skin entry necessary to perform the procedure
• Active systemic infection
• Coagulopathy
• Allergy to infused medication
• Immunocompromised state that would preclude adequate healing after implantation of the permanent device
PERIOPERATIVE CONSIDERATIONS FOR TRIALING
Preoperative Considerations
• When possible, a patient should be weaned off, or reduced to a minimum amount of oral or transdermal opioid prior to an intrathecal drug delivery system trial.
• Preadmission testing should include screening for coagulopathies with a PT/INR, PTT, and bleeding time.
• Additional history should be taken to screen for undiagnosed coagulopathies such as von Willebrand disease and history should include any previous history of unusual bleeding due to surgeries or lacerations.
• Anticoagulants should be discontinued as per ASRA guidelines prior to performance of needle-based spinal procedures. Additionally, it is recommended that clinicians consider stopping aspirin for 1 week prior to the procedure, and herbal supplements such as fish oil that may cause bleeding for 2 weeks preoperatively.
• Preadmission testing should screen for any electrolyte abnormalities or cardiovascular risks that would preclude a patient from undergoing a minor surgical procedure.
• Anatomical considerations such as existing hardware, skin integrity, skin masses, soft tissue masses, tumor growth, patient tolerance to positioning and body habitus should be evaluated preoperatively in order to determine the feasibility of needle placement at the desired location.
• Please refer to Figure 67-1.
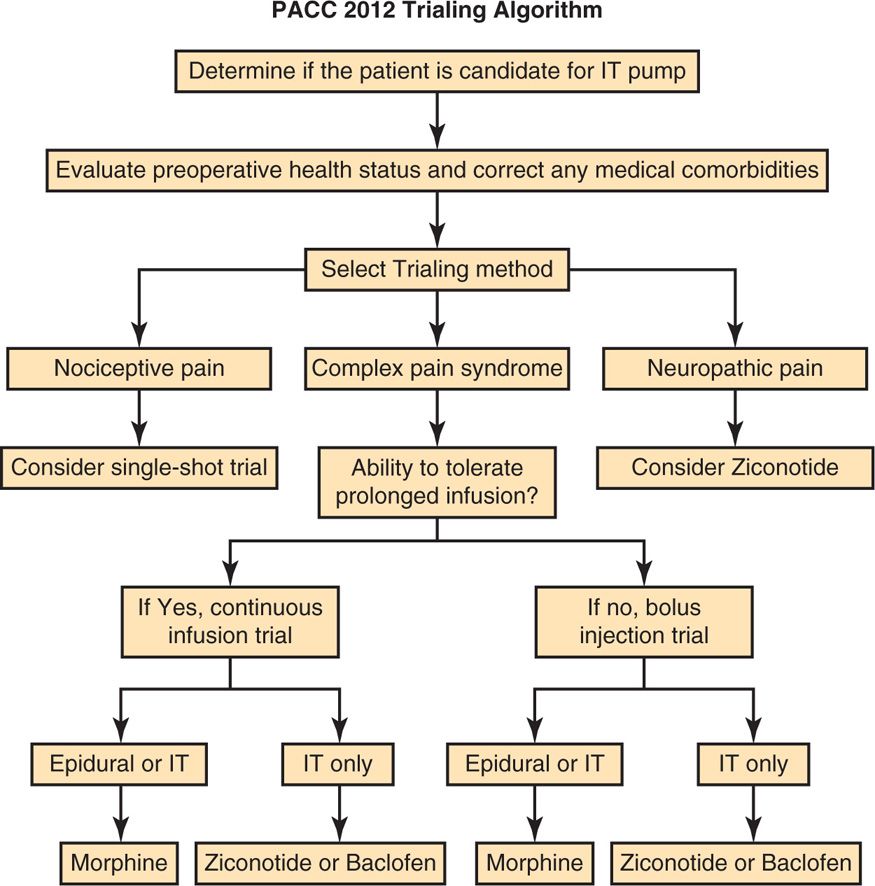
Figure 67-1. PACC 2012 trialing algorithm.
During The Trial
• Preoperative administration of IV antibiotics in high risk patients may be considered for placement of catheters for trialing. The choice of antibiotic should be determined with evaluation of common pathogens in the clinician’s region. Infectious disease specialists may be helpful in making recommendations for specific antibiotic choice and dose in each region.
• Oral or transdermal opioids should be reduced or discontinued when feasible in order to best assess the efficacy of epidural or intrathecal infusion of opiates.
• Dosing epidural versus intrathecal: it should be noted that intrathecal dosing is generally 1:10 relative to epidural dosing of opiates. Similar ratios apply for bupivacaine, baclofen, and clonidine.
• It is recommended that continuous pulse oximetry be utilized during continuous epidural or intrathecal infusions.
• Vital signs should be assessed at 4-hour intervals.
• Any signs of sedation, confusion, low oxygen saturation, or hypotension should prompt discontinuation of the infusion until the physician is contacted for instruction.
• Patients should be monitored closely for development of allergy to the infused agent for the first 2 hours after initiating infusion.
• Patients should resume pretrial doses of oral or transdermal opiates after the infusion has stopped at discharge, or should be tapered off infused opiates prior to discharge if on no oral opiates at home.
• Blood pressure should be monitored for rebound hypertension for 24 to 48 hours subsequent discontinuation of clonidine infusion.
• If an opiate is combined with ziconotide, all psychological complications that could occur with administration of ziconotide would apply as described in the ziconotide trialing section.
Mechanics of Fluoroscopic Imaging
• After positioning the patient on the table, ideally in a prone position, a “true” anterior-posterior (AP) image of the lumbosacral spine is performed. Cephalocaudal tilt is utilized to image the inferior end plate of the vertebral body superior to the interlaminar space targeted. It is recommended to “square off” the end plate of this vertebral body. A true AP image should visualize the spinous process at that level as centered between the adjacent pedicles.
• Reduction of lumbar lordosis with padding or pillows placed under the abdomen, when feasible, will help increase the interlaminar space available the desired entry level in the upper lumbar spine.
• Lateral views should be obtained at this level prior to patient prep in order to be sure that adequate visualization of the needle tip or catheter will be feasible in the patient’s position.
• Typical interlaminar location for insertion of the intrathecal needle tip should be L2-L3 or L3-L4.
• Choosing a level below L2 or the level of the conus is suggested for intrathecal placement of needles when feasible.
PARAMEDIAN TECHNIQUE FOR INTERLAMINAR PLACEMENT OF NEEDLE
This preferred technique is utilized for single-shot trials as well as catheter placement for continuous infusion, although it may be performed at different spinal levels depending on the specific technique. This procedure applies to trialing whether for pain or spasticity.
• A paramedian technique for interlaminar placement of the needle tip is suggested with lateral to medial, caudad to cephalad, and posterior to anterior angling is utilized.
• Fluoroscopic identification of the interlaminar space to be targeted and appropriate imaging of the targeted level (as described under fluoroscopic views) is performed first.
• A needle insertion site is identified under fluoroscopy at approximately 2 vertebral body segments inferior to the targeted interlaminar level with the medial border of the pedicle at that level used as a reference point for needle insertion.
• Lidocaine is applied at the overlying skin surface with the 25-gauge 1.5-in needle and 5 to 10 cc of 1% lidocaine. A skin wheal is created and the subcutaneous tissue is infused with lidocaine.
• A 15-blade scalpel is used to make a small stab incision at the site of needle insertion.
• The Tuohy needle is advanced through the anesthetized skin with AP imaging visualizing the advancement in a lateral to medial, caudad to cephalad, posterior to anterior trajectory until the tip of the Tuohy needle reaches the laminae of the vertebral level that lies along the inferior ipsilateral side of the interlaminar space targeted.
• The needle angle utilized should be a shallow angle of 30 degrees or less, relative to the spine (Figure 67-2).
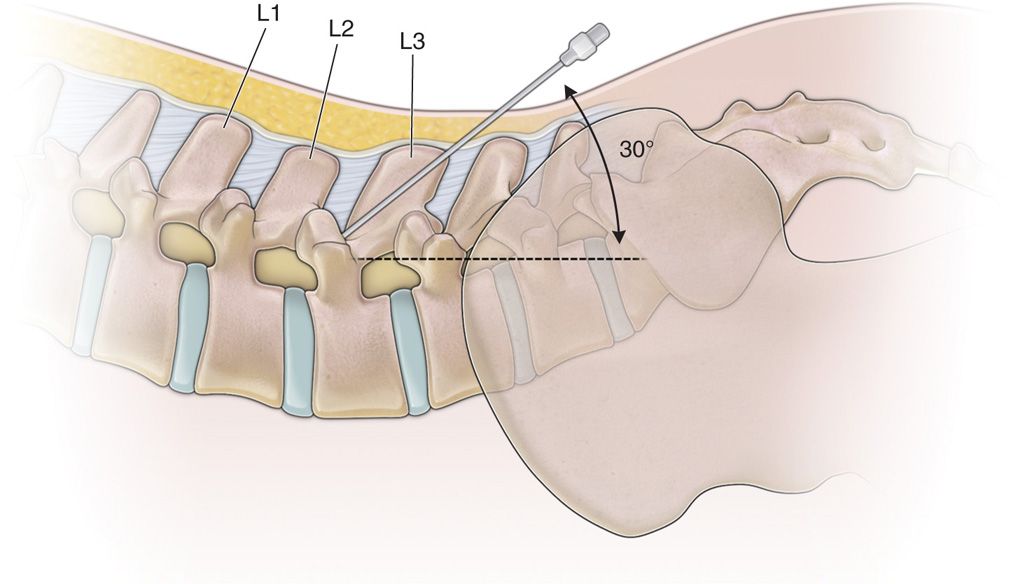
Figure 67-2. Shallow needle placement for paramedian technique.
• Adjustments to needle entry location may vary based on body habitus in order to maintain this angle such that in individuals with more adipose tissue the needle entry point may need to be more than 2 segments inferior to the targeted site and the needle tip may lie as far lateral as the lateral edge of the pedicular line and the inverse is true for very thin individuals.
• The needle should be guided directly to this bony landmark and should touch bone before being further advanced.
• A lateral image to ensure appropriate location of the needle tip along the laminae in this position is suggested (Figure 67-3).
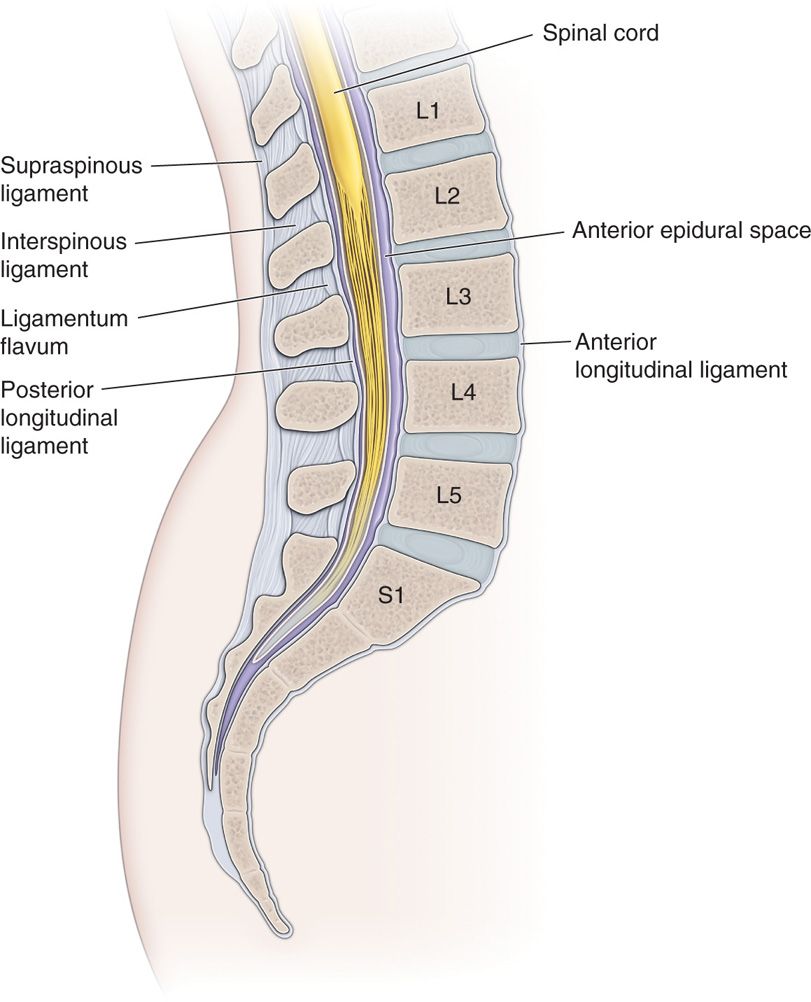
Figure 67-3. Lateral image diagram of lumbar spine anatomy.
• Once the needle tip is confirmed to be in this location, the needle is subsequently “walked off” of the laminae and is advanced through the ligamentum flavum into the epidural space with loss of resistance technique using the Luer-slip syringe with 2 to 3 cc of normal saline in the loss of resistance syringe, as well as fluoroscopic visualization confirming midline spinal placement in AP view and placement of the depth of the posterior epidural space on lateral view (Figure 67-4).
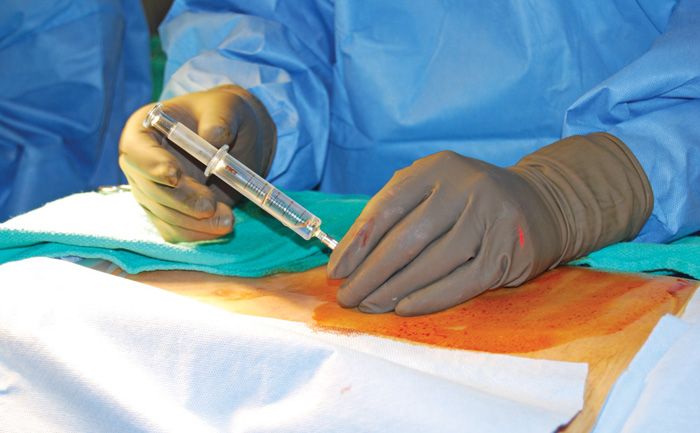
Figure 67-4. Loss of resistance.
• Lateral imaging can further confirm the location of the needle depth. Infusion of contrast dye could be utilized to confirm posterior epidural spread, but is not always necessary in cases where intrathecal needle tip placement is desired.
• This is the final needle position for epidural injection or epidural catheter placement.
For advancement to the intrathecal space:
• The needle is then advanced through the dura mater into the intrathecal space for single shot intrathecal injection or for the purpose of intrathecal catheter placement.
• Placement into the intrathecal space should be confirmed by spontaneous flow of CSF via direct visualization or aspiration and may be confirmed by visualization of the needle tip in the central spinal canal on lateral imaging.
INTRATHECAL PUMP INFUSION SYSTEM TRIAL FOR PAIN
Intrathecal pump infusion system trials for pain are performed preceding permanent pump placement to assess patient efficacy and tolerability. Both pain reduction and functional improvement measures allow the clinician to determine efficacy and patient tolerability may be determined by both subjective measures from the patient’s perspective and objective measures from the clinician’s perspective.
The objective measures include:
• The patient’s respiratory status and oxygenation
• Blood pressure stability
• Urinary status
• Bowel function status
• Physical functioning status with respect to strength and agility
• Absence or presence of allergic reaction to the medications delivered
Continuous infusion trial:
• Trialing has traditionally been performed in multiple formats.
• Some clinicians propose intrathecal trials, as they best simulate a permanent system via delivery of the medications to the same spinal space.
• The potential negative adverse effect of performing continuous intrathecal trials is the high likelihood of postdural puncture spinal headache that occurs upon discontinuation and removal of the trial catheter from the intrathecal space.
• Other clinicians prefer continuous epidural infusion. Continuous epidural infusion does simulate intrathecal pump system infusion.
• Clinically, it appears that there is no significant difference in outcomes between continuous intrathecal and continuous epidural trial infusion and both simulate the permanent system with equal safety and efficacy.
• One exception is the medication ziconotide, which, due to its high molecular weight, does not appear to cross over from the epidural space into the intrathecal space and hence requires intrathecal trialing to simulate its potential efficacy with respect to permanent pump placement.
Single-shot trialing:
• Finally, some clinicians prefer single-shot trialing for opiate trials.
• Single injections of morphine have been traditionally shown to be successful predictors of efficacy in permanent systems.
• While intuitively the application of continuous administration of opiate via epidural or intrathecal infusion may seem superior in detecting efficacy with respect to pain relief and improved function in a patient whose pain may fluctuate within the day or on a day-to-day basis.
• The 2007 Polyanalgesic Consensus Conference (PACC) recommendations for pump trialing suggest that there is a lack of comparative data in the literature to determine superiority of continuous trialing of opiate versus single injection trialing.
• While the current trend is to look at functionality as a predictor of efficacy with scales such as the Oswestry Disability Index, this can be challenging in the short period of time available for assessment that is present during a single injection trial.
PATIENT SELECTION
Patients with chronic pain generally fall into 1 of 2 categories of pain types as defined by Dr Elliot Krames. Most patients suffer from a predominance of either neuropathic pain or nociceptive pain.
• Those who suffer primarily from nociceptive pain generally respond more favorably to opioid medications and thus are trialed with morphine when possible.
• Those who primarily suffer from neuropathic pain may respond better to nonopioid agents and thus are often trialed with ziconotide.
• The specific mechanism of trialing may vary based on the medications being utilized. Continuous infusion of opioids for the purpose of trialing can be done epidurally or intrathecally.
• The advantage of continuous epidural infusion trialing is the lack of postdural puncture headache that occurs frequently after removal of the catheter in continuous intrathecal trials.
INTRATHECAL MEDICATIONS FOR PAIN
The FDA has approved the intrathecal use of morphine, ziconotide, and baclofen for use in humans.
• Morphine is mostly effective in individuals with nociceptive pain conditions.
• Ziconotide is primarily effective in patients with a predominance of neuropathic pain.
• Baclofen: Severe spasticity can be a painful phenomenon, and the use of intrathecal baclofen may therefore provide pain relief in those with painful spasticity.
NON-FDA-APPROVED MEDICATIONS
Consensus guidelines for the use of intrathecal medications such as those coming from the Interdisciplinary Polyanalgesic Consensus Conference in 2007 advocate the judicious use of certain non-FDA-approved medications for individual use or in combination with FDA-approved intrathecal medications or as single agents as follows:
• Hydromorphone, fentanyl, and sufentanil—any one of these individual opioids may be used alone or in combination with other suggested nonopioid agents in the case of lack of efficacy or tolerability to morphine.
• Bupivacaine—this anesthetic is utilized for neuropathic pain generally as an adjunct to an intrathecal opioid such as morphine.
• Clonidine—this intrathecal agent is often utilized as an adjunct to an intrathecal opioid such as morphine and is utilized for neuropathic pain conditions.
Clinical pearls for the use of non-FDA-approved suggested intrathecal agents:
• Hydromorphone—is often dosed at 10% of the corresponding effective dose of morphine. It has a side effect profile similar to morphine.
• Fentanyl—this lipophilic agent is an opioid with intrathecal potency approximately 100 times that of intrathecal morphine.
• Sufentanil—this highly lipophilic agent is approximately 10 times more potent than fentanyl. Fentanyl has similar chemical characteristics and a similar side effect profile to that of sufentanil. Both sufentanil and fentanyl, due to their highly lipophilic state, tend to provide pain relief at only 1 or 2 vertebral segments above or below the catheter tip and are, therefore, most effective in patients who do not have widespread pain. Anatomic localization of the catheter becomes more critical in patients who are known to have intolerability to morphine or hydromorphone and are expected to be utilizing fentanyl or sufentanil in their permanently implanted system.
• Bupivacaine—weakness or urinary dysfunction are often associated with epidural or intrathecal bupivacaine infusion. Patients receiving bupivacaine should be monitored for these side effects. These side effects are reversible upon the discontinuation of the bupivacaine.
• Clonidine—this drug potentially has opioid sparing effects in its treatment of neuropathic pain and may reduce blood pressure. Patients should be carefully monitored for hypotension. Just as with discontinuation of clonidine orally, discontinuation of clonidine infusion can lead to hypertension, withdrawal or more serious complications, and should be titrated downward prior to complete discontinuation.
• Baclofen—if continuous baclofen infusion is utilized in a trial setting, the infusion should be weaned downward rather than discontinued abruptly due to the risk of seizure with abrupt baclofen discontinuation.
• Other agents—other agents utilized in an intrathecal pump infusion system.
• Injectable saline—may be utilized for flushing of the pump or catheter and can be utilized for continuous infusion.
TRIALING VIA CONTINUOUS EPIDURAL INFUSION OF OPIATES
Equipment for needle placement (Figure 67-5):
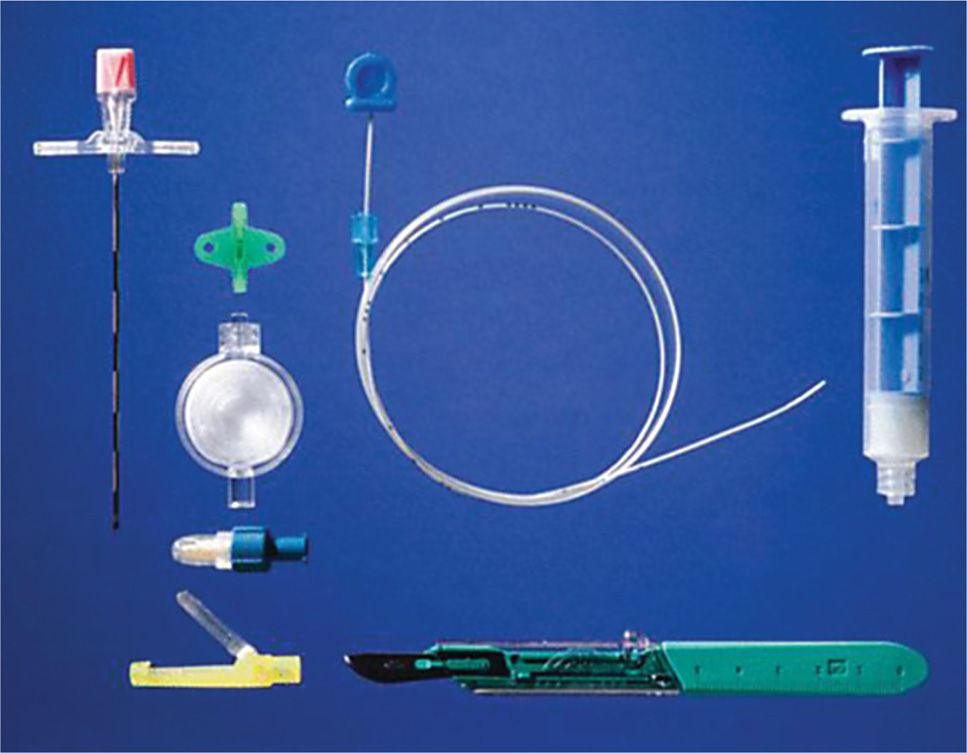
Figure 67-5. Tools for trialing: Tuohy needle, syringe, filer, and cap. (Reproduced with permission from MILA International, Inc.)
• Tuohy needle—16- or 17-gauge 3- to 6-in (based on body habitus)
• 25-gauge 1.5-in needle
• 10-cc syringe for local anesthetic medications
• 5-cc syringe for contrast dye
• 10-cc syringe for medication infusion
• Luer-slip loss of resistance syringe (10 cc)
• Connector (extension) tubing with stop cock
• Sterile preparation materials
Equipment for continuous epidural infusion (all of the above plus):
• Epidural catheter connector piece
• Epidural catheter 1 size smaller than the gauge of the Tuohy needle
• Antibacterial filter
• 1 catheter end cap
• Tunneling device
• Steri-strips
• Skin preparation
• Antimicrobial agent (iodine povacrylex and isopropyl alcohol or chlorhexidine gluconate may be used)
PROCEDURE OF NEEDLE PLACEMENT
• Needle placement will be performed with a Tuohy needle via the technique described as “Paramedian Technique for Interlaminar Placement of Needle.”
• After placement and confirmation of needle placement in the posterior epidural space via the “Paramedian Interlaminar Needle Placement Technique,” an epidural catheter is directed through the spinal needle and guided through the posterior epidural space in a caudad to cephalad trajectory.
• Lateral imaging on fluoroscopy can confirm that the catheter remains posterior relative to the spinal canal and AP imaging under continuous or intermittent views allows steering of the catheter from a medial to lateral perspective as the catheter advances in a cephalad direction to its desired location (Figures 67-6 and 67-7).
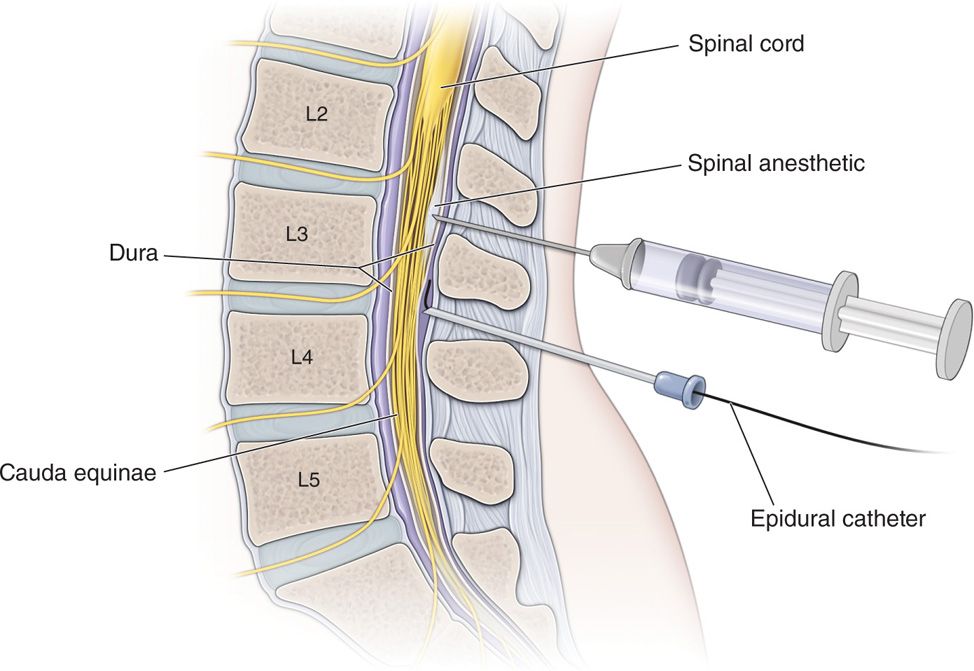
Figure 67-6. Epidural trial.
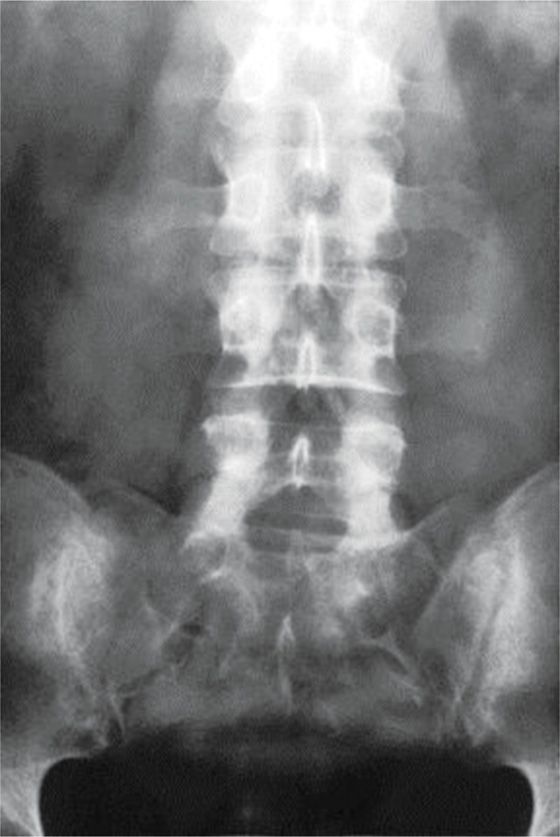
Figure 67-7. AP image.
• After the catheter tip is placed at its desired location, the catheter should be checked for flow and catheter integrity by placing the connector and filter onto the end of the catheter and infusing 1 to 2 cc of contrast dye (or saline if there is a dye allergy).
• Lateral imaging should always be performed at this point to confirm that the catheter tip is indeed in the posterior epidural space.
• The catheter tip should be located medial to the medial pedicular line in order to avoid placement adjacent to the spinal nerve root at that segment.
• Then, the connector and filter must be unattached for needle removal and/or if tunneling is desired (Figure 67-8). If tunneling is desired, it would be performed at this step as described in the above section titled “Tunneling of Catheters for Trialing.”
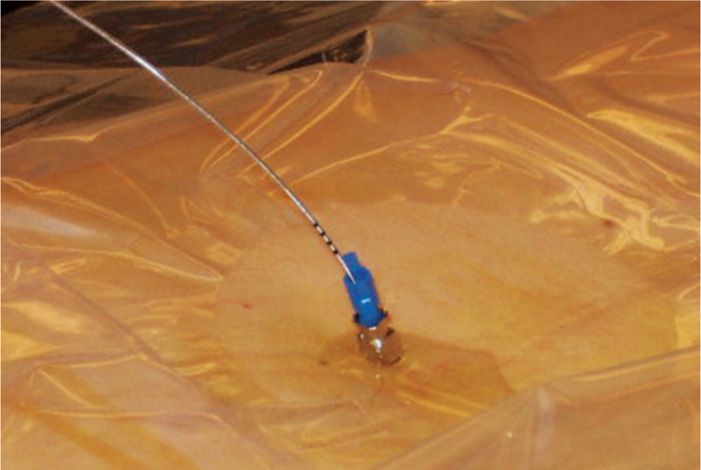
Figure 67-8. Epidural needle placement with catheter.
• When tunneling is completed and the Tuohy needle has been removed, then the catheter is confirmed to be in its desired location by a final AP fluoroscopic image.
• Next, the catheter connector and filter are placed.
• Flush the catheter with a small volume solution, such as saline, to ensure catheter integrity, and an end cap is placed until epidural infusion of opioid or combined agents is initiated.
Typical desired locations may be as follows:
• T10: Commonly utilized for back and leg pain from FBSS
• Sacrum: Common site for catheter placement for pelvic or rectal pain
• C3 or C4: Common location for catheter placement for head and neck pain
• T1: Often used for placement for mid-chest pain
Clinical pearls for catheter tip placement:
• Placement of the tip below the conus reduces the risk of spinal compression when intrathecal granulomas develop.
• Lipophilic opioids such as fentanyl or sufentanil may not spread more than 1 or 2 vertebral segments above or below the catheter tip and therefore will not control head and neck pain as well as back and leg pain in the same individual.
TRIALING FOR OPIATES VIA CONTINUOUS INTRATHECAL ADMINISTRATION OF MEDICATION
The efficacy of continuous epidural and continuous intrathecal trials with opiates appears to be similar based on the literature review. The administration of opiates via continuous intrathecal infusion requires significantly lower doses compared to epidural infusion and carries with it the possibility of postdural puncture headaches and meningitis, both which tend to occur at a higher rate than with continuous epidural infusion of opiates.
Procedure for Catheter Placement
• A Tuohy needle is placed via the “Paramedian Technique for Interlaminar Placement of Needle.”
• Once the needle is advanced through the dura and into the intrathecal space and free CSF flow is observed, then an intrathecal catheter is passed through the needle into the intrathecal space and the tip is steered to its desired location with the tip found to be medial to the medial pedicular line on AP fluoroscopic imaging and localized in the spinal canal on lateral fluoroscopic imaging.
• If tunneling is to be performed, it would be performed via the technique described in “Catheter Tunneling Technique for Trials.”
• After the desired catheter location is obtained and the Tuohy needle is removed, the catheter is checked for flow integrity with the catheter connector and filter attached.
• Then, either the end cap is placed until intrathecal opioid administration is initiated.
• A 1- to 2-mL flush with normal saline of the catheter may precede end cap placement.
Single Shot Injection Opiate Trial
Some clinicians prefer single injection opiate trials to continuous infusion intrathecal or epidural infusion.
• The advantage of a single injection is that there is potentially less risk of infection due to the lack of an indwelling catheter.
• The obvious disadvantage is the short period of time available to make a comprehensive assessment, not only of the patient’s response to pain, but also their potential improvement in functionality.
• The growing interest in assessing function: including gait and mobility, transfers including movement into and out of the bed and chair, necessitates the importance in evaluating the response to spinal delivery of opioids over a greater period of time in order to thoroughly assess function based on scales such as the Oswestry Disability Index.
• Furthermore, since some patient’s pain fluctuates, not only throughout the course of a given day, but from day to day, there may be some significant benefit to monitoring a patient’s response to pain over a prolonged period of time (in days) with continuous epidural or intrathecal infusion of opiate.
Single Injection Trial Procedure for Opiate (Morphine)
• Tuohy needle is placed in the lumbar spine below the level of L2 (most typically at L2-L3 or L3-L4).
• Advance the needle into the intrathecal space via the technique outlined in “Paramedian Technique for Interlaminar Placement of Needle.”
• Once achieving free flowing CSF, an intrathecal injection of morphine is performed. Dosing varies greatly in the literature and is dependent on both the patient’s tolerance to opiates and pain demand.
• Resuscitation equipment, including oxygen and medications, should be immediately available in the case of respiratory depression induced with a morphine injection.
• The patient should be monitored for several hours with respect to the potential positive effects of the injected morphine, such as reduction in pain on a numeric pain score or visual analog score.
• The patient should also be assessed as to changes in functionality and comparison should be made between preinjection gait, transfers and strength as compared to postinjection gait, transfers and strength.
• Monitoring for adverse events and side effects should also be performed for several hours postinjection.
• Common side effects would include those typical with administration of morphine, such as nausea, vomiting, dizziness or lightheadedness, respiratory depression, sedation, confusion, somnolence, and urinary retention.
• Itching is a common side effect and rash may be indicative of an allergy to the medication.
Post-Trial Considerations (for continuous epidural or intrathecal infusions or single shot method)
• Respiratory depression may occur.
• Respiratory status should be closely monitored with oxygen at the bedside and pulse oximetry monitoring.
• Meningitis may occur with intrathecal or epidural trialing and is most prevalent with intrathecal trials and has been reported with single injection trials.
• Nausea and/or vomiting is a frequent side effect of opiates.
• Drowsiness or somnolence may occur.
• Blood pressure should be closely monitored as hypotension is a frequent side effect.
• Urinary retention may occur.
• Seizures and/or coma are an uncommon occurrence.
• Postoperative antibiotics during the course of injection are debatable and are not currently supported by the scientific literature.
Other Adverse Events
• Bladder or bowel changes
• Paralysis
• Death
• Spinal headache
• Epidural hematoma
• Epidural abscess
• Intrathecal hematoma
• Intrathecal abscess
• Discitis
• Injury to adjacent nerve root
• Skin infection including cellulitis and abscess
• Skin erosion
• Skin irritation or allergy to skin preparation
• Anaphylaxis from any medications utilized
• Allergy to hardware or other supplies utilized
• Catheter dislodgement (with continuous infusion)
TRIALING WITH ZICONOTIDE
Intrathecal trialing, as compared to epidural trialing of ziconotide, is necessary due to the lack of transportation of the ziconotide molecule from the epidural to the intrathecal space, believed to be due to its large molecular size and hydrophilic state of the molecule. Intrathecal trials are typically performed via continuous infusion.
• The continuous infusion is proposed to be the superior trialing method by Dr David Caraway and Dr Michael Saulino.1
• However, authors such as Dr Eric Grigsby,2 suggest that single-shot ziconotide trials are equally predictive of long-term success of permanent pump implantation for ziconotide administration.
• According to Dr Allen Burton, et al,4 when comparing 3 methods of ziconotide trialing: continuous infusion, bolus injection, and limited duration infusion, all 3 methods appear to be effective in predicting patient response to long-term administration of ziconotide therapy and none prove to have superior safety or efficacy given current scientific data.4
• Intrathecal ziconotide was developed with the purpose of treating opiate resistant neuropathic pain.
• It is a neuroactive peptide isolated from the conus magus snail, which appears to have efficacy in many neuropathic pain conditions.
• It has been studied in FDA trials as a single agent and has more recently been studied in combination with opiates for continuous infusion as seen by a study by Dr Timothy Deer, et al.3
Continuous Intrathecal Ziconotide Infusion
Continuous intrathecal infusion of ziconotide has been the most frequently reported method of trialing in the literature to date. While starting doses of ziconotide for continuous infusion have varied from study to study, a trend toward side effects occurring at higher infused doses has been identified.
• Most recently, Caraway et al have suggested a starting dose of 1.2 μg/d as compared to 2.4 μg/d as seen in multiple previous trial results published.
• Dr Caraway’s 3-day titration schedule allows for a dose increase of 1.2 μg every 12 to 24 hours as determined to be necessary by the clinician.
• Careful monitoring for side effects or adverse events is suggested.
• Typical ziconotide trials are performed in the hospital.
• Psychosis is a reversible, yet severe, potential side effect that may occur with escalating doses of ziconotide and requires discontinuation of the trial.
• Management of the complications of psychosis, which may include lack of verbal response or immobilized state, often includes admission to a psychiatric unit.
• The psychosis may take days to weeks to resolve completely.
• Other frequent side effects include nausea and vomiting (approximately 50% in some studies), alterations in gait, somnolence, memory impairment or aphasia, and urinary retention.
• There does not appear to be any withdrawal symptoms with abrupt termination of ziconotide.
• It should be noted, however, that patients undergoing intrathecal trialing for ziconotide should either be weaned off of their opiates preceding the trial or continued on their regular dose of opiate via oral or transdermal administration during the course of the trial, so as to avoid opiate withdrawal.
• There does not appear to be any significant respiratory depression associated with ziconotide infusion.
Equipment for Needle Placement (as above in this chapter)
Procedure
• Placement of the Tuohy needle as described in “Paramedian Technique for Interlaminar Placement of Needle” is performed first.
• After the needle is advanced into the intrathecal space, and CSF flow is obtained, the stylet is removed, and the catheter is steered into the desired intrathecal location.
• If tunneling is to be performed, it should be performed as described in the section titled “Catheter Tunneling Technique for Trials.”
• After completion of catheter placement, and ensuring proper catheter location and function, intrathecal ziconotide infusion may begin.
• Trial length typically consists of 3 days or more of inpatient monitoring.
• Determination of efficacy is assessed by both improvements in pain and/or function.
• Monitoring includes nursing assessment of vital signs. Pulse oximetry if on opiates, mental and cognitive status, urinary output and neurologic checks.
• Monitoring in an inpatient setting should occur at a minimum of 4 hour intervals.
• Adjustments in ziconotide dosing during the trial phase should be considered no more frequently than at 12 hour intervals.
• Slow upward titration appears to decrease the likelihood of significant side effects as most ziconotide side effects appear to be dose dependant.
• After discontinuation of the trial, and removal of the catheter, the patient should be monitored for postdural puncture headaches.
• Adverse events with intrathecal placement of catheters may occur and are similar to those referenced under the section “Post-Trial Considerations” in the above opiate trialing section.
Limited Duration Infusion Trials of Ziconotide
A limited duration trial involves infusion of ziconotide via an external pump and is generally performed over the course of a 1-hour time period.
• Typical infusion doses vary between 1 to 10 μg infusion of ziconotide into the intrathecal space during this time frame.
• The patient is then assessed for response to the ziconotide via reduction in pain or improvement in function over the following 4 hours.
• As with continuous infusion, monitoring for known side effects is imperative.
• The positive or negative effects of ziconotide from a limited duration infusion may persist for greater than 24 hours.
• Limited evidence in the literature is available to suggest a specific length of time for which inpatient or outpatient monitoring via the physician should be performed.
Equipment for Needle Placement (as above in this chapter)
• Externalized pump
Procedure for Continuous Infusion of Ziconotide
• Patients should be weaned from all spinal drugs prior to initiation of the infusion.
• Placement of a Tuohy needle via the “Paramedian Technique for Interlaminar Placement of Needle.”
• Once the needle tip is advanced into the intrathecal space, and CSF flow is obtained, then the catheter is placed into the needle and steered into the desired location.
• If catheter tunneling is to be performed, it is performed as described in “Catheter Tunneling Technique for Trials.”
• After catheter placement is completed and the function and continuity of the catheter has been confirmed, then the limited duration infusion with an external pump and ziconotide dose typically varies between 1 to 10 μg intrathecally over the course of 1 hour.
• After the 1-hour infusion, the intrathecal catheter is removed manually and patient monitoring continues for a suggested minimum for 4 hours with the understanding that positive or negative effects of ziconotide may persist for 24 hours.
• Any side effects or adverse events that are observed during the 4-hour monitoring period would preclude discontinuation of patient monitoring until these effects subside.
Bolus Trials With Ziconotide by Single or Multiple Injections
• The obvious advantage of bolus trials involves the lack of necessity of an indwelling catheter for any period of time.
• Bolus trials do appear to offer adequate efficacy with respect to the predictive value of response for permanent pump placement as suggested by Dr Grigsby.
• Rosenblum et al reported in a randomized, double blind, placebo controlled trial, administration of up to 4 intrathecal ziconotide injections with placebo being one potential injected substance.
• Other studies by Dr Grigsby involve administration of a single injection of 1 μg of intrathecal ziconotide with visual analog scores assessed prior to the injection and 1 hour after the injection as well as follow-up assessment occurring 24 hours after the injection.
• Subjective patient data was also collected to determine their satisfaction with pain relief as perceived after the injection.
• Some patients in this trial who failed to achieve efficacy at 1 μg were later injected with 3 or 5 μg doses.
• Monitoring included visual analog pain scores and significant reductions in pain combined with a lack of intolerable side effects yielded a successful trial outcome.
Equipment for Single Bolus Trial (as above in this chapter)
Procedure of Single-Shot Trial of Ziconotide
• Place Tuohy Needle via the “Paramedian Technique for Interlaminar Placement of Needle.”
• Once the needle tip is advanced into the intrathecal space and free CSF is obtained, injection of the ziconotide bolus is performed (bolus dosing in the literature varies between 1 μg and up to 50 μg with typical doses between 1 and 5 μg).
• After bolusing, the needle is removed.
• The patient is carefully monitored for both efficacy and side effects with monitoring performed typically over the first hour after the injection, and effects from a single bolus are often monitored for as long as 24 hours postinjection.
• The potential for side effects or adverse events does not differ from those potentially occurring with limited dose infusion or continuous infusion of ziconotide via catheter placement, with the exception that catheter dislodgement could not happen and that the risk of infection or meningitis should be minimal compared to risk seen with catheter placement.
POST-TRIAL CONSIDERATIONS (FOR CONTINUOUS INTRATHECAL INFUSIONS OR SINGLE-SHOT METHOD)
• Psychosis commonly occurs during administration of ziconotide.
• Meningitis may occur with intrathecal trialing and has been reported with single injection trials.
• Nausea and/or vomiting is a frequent side effect.
• Drowsiness or somnolence may occur.
• Blood pressure should be closely monitored.
• Urinary retention may occur.
• Postoperative antibiotics during the course of injection are debatable and are not currently supported by the scientific literature.
Other Adverse Events
• Bladder or bowel changes
• Paralysis
• Spinal headache
• Epidural hematoma
• Epidural abscess
• Intrathecal hematoma
• Intrathecal abscess
• Discitis
• Injury to adjacent nerve root
• Skin infection including cellulitis and abscess
• Skin erosion
• Skin irritation or allergy to skin preparation
• Anaphylaxis from any medications utilized
• Allergy to hardware or other supplies utilized
• Catheter dislodgement (with continuous infusion)
INTRATHECAL BACLOFEN PUMP TRIALING FOR SPASTICITY
Intrathecal baclofen pump implantation is utilized for patients with severe spasticity. Patient selection for this modality includes those with spasticity who have one or more of the following criteria:
• Inadequate management of spasticity with physical modalities and oral spasticity agents.
• Intolerance to side effects associated with oral spasticity agents and failure of alternate modalities.
• Widespread, nonlocalized spasticity that cannot be managed with nonpharmacological modalities.
• Patient positioning challenges created by the spasticity.
Preoperative Considerations
• Informed consent with discussion of all possible risk or adverse events associated with the procedure.
• Preoperative planning of patient positioning based on their anatomy, degree of spasticity, and ability to maintain prone positioning as some individuals may need to be trialed in a lateral decubitus position.
• Evaluation for allergy to baclofen, contrast dye or any of the components utilized in performing the procedure or inherent in the system to be implanted.
• IV access and equipment for resuscitation including appropriate medications and oxygen should be immediately available.
• Anticoagulation considerations should be discussed with the patient and dealt with in the preprocedure clinical assessment.
• Body habitus should be assessed to determine the appropriate length of spinal needle necessary to perform the procedure.
• Ashworth assessment on admission.
• For intrathecal shot trialing, the lumbar level chosen should be below the level of the conus (below L2).
• Preoperative antibiotics should be considered for any patient with a presumed infection or infectious risk or those undergoing catheter placement for continuous infusion.
• Urinary retention may worsen with intrathecal baclofen administration.
Equipment for Single-Shot Infusion (as above in this chapter)
Medications Used
• Baclofen 25, 50, 75, or 100 μg (dose chosen based on patient’s degree of spasticity and tolerance to oral baclofen)
• 1% lidocaine
• Injectable saline
• Nonionic water-soluble contrast iopamidol
Considerations for Single-Shot Baclofen Injection
• Contraindications to iopamidol include:
![]() Hypersensitivity to iodine containing preparations
Hypersensitivity to iodine containing preparations
![]() Hypersensitivity to iodine contrast media
Hypersensitivity to iodine contrast media
![]() Severe renal disease
Severe renal disease
![]() Multiple myeloma
Multiple myeloma
![]() Waldenstrom macroglobulinemia
Waldenstrom macroglobulinemia
![]() Pregnancy
Pregnancy
![]() Postoperative antibiotics generally not needed for single shot trials
Postoperative antibiotics generally not needed for single shot trials
Needle Placement Technique
• A paramedian technique for interlaminar placement of the needle tip is suggested with lateral to medial, caudad to cephalad, and posterior to anterior angling is utilized as described in the section “Paramedian Technique for Interlaminar Placement of Needle.”
Technique for Delivery of Baclofen in Single-Shot Trials
• Administration of the 25, 50, 75, or 100 μg baclofen dose (50 μg most commonly utilized) through the Tuohy needle with connector tubing.
• Pretrial and postinfusion monitoring of spasticity utilizing the Modified Ashworth Scale.
• Careful documentation of spasticity changes after administration of baclofen as well as evaluation of gait, transfers and posture, and pre- and postinjection.
• Spasticity monitoring at 2 and 4 hours post single-shot dosing is commonly performed with careful documentation of the above at these intervals.
• A 2-point reduction on the Modified Ashworth Scale with tolerability of the intrathecal baclofen is generally considered favorable in assessment of the overall success of the trial.
Technique for Placement of Intrathecal Catheter for Continuous Baclofen Infusion
First, needle placement as described in the section “Paramedian Technique for Interlaminar Placement of Needle.”
• An epidural catheter of one size smaller than the needle gauge (one gauge number higher) should be placed through the Tuohy needle (with stylet removed).
• Fluoroscopic visualization of the catheter initially exiting the tip may be assessed best in lateral view.
• Advancement of the catheter under AP view allows steering medially and laterally.
• The final position of the catheter tip should be at the midline and is often placed at the thoraco-lumbar junction, although catheter tip placement may vary based on desired outcome.
• If tunneling of the catheter is desired, then the Tuohy needle should be left in place until tunneling is performed.
Catheter Tunneling Technique for Trials
• The desired location for the tunnel exit site should be prepped and anesthetized with 1% lidocaine and the projected course of the tunneled catheter should be anesthetized with lidocaine subcutaneously.
• The projected exit location of the tunneled catheter is usually positioned several inches lateral and slightly inferior to the Tuohy needle site.
• A small stab incision with a 15-blade scalpel at the projected exit site of the tunneled catheter may be placed into the anesthetized skin prior to the tunneling device introduction.
• The tunneling device may then enter through this stab incision and is directed subcutaneously, just below the dermis, through the projected tunnel and over to the Tuohy needle.
• The tunneling device should then be directed so that the sharp end of the device exits the stab incision made for the Tuohy needle.
• The sharp end of the tunneling device then lies adjacent to the Tuohy needle where the needle enters the skin.
• The sharp center hardware of the tunneling device is then extracted from the outer sheath of tunneler.
• Carefully remove the Tuohy needle while maintaining slight forward pressure on the catheter so as not to withdraw it from its desired location.
• Guide the catheter from the Tuohy needle skin site through the sheath of the tunneler and through the exit site.
• Once the catheter tip extends beyond the tunnel exit site, pull the outer sheath of the tunneling device out of the tunnel—leaving the catheter within the tunnel.
• Pull any additional catheter slack outward so that the catheter is taut and the entire catheter is positioned below the skin level through its course over to the exit site.
• Close both 15-blade stab wound sites with steri-strip.
• Proper catheter flow should then be tested by placing the catheter hardware connector and filter onto the catheter end and then infusing injectable saline or contrast of 1 to 2 cc thereby ensuring proper flow into the epidural space.
• The use of contrast infusion for catheter integrity testing may be considered to ensure proper flow and ensure that the catheter is free of any leaks.
• The catheter should then be flushed with saline and the filter end capped until the intrathecal baclofen continuous infusion is initiated.
Post-Trial Considerations
• Respiratory depression may occur.
![]() Respiratory status should be closely monitored with oxygen at the bedside and pulse oximetry monitoring.
Respiratory status should be closely monitored with oxygen at the bedside and pulse oximetry monitoring.
• Meningitis may occur with intrathecal or epidural trialing and is most prevalent with intrathecal trials.
• Nausea and/or vomiting is a frequent side effect of intrathecal baclofen administration.
• Drowsiness or somnolence may occur.
• Blood pressure should be closely monitored as hypotension is a frequent side effect of intrathecal baclofen administration.
• Urinary retention may occur.
• Seizures and/or coma may occur.
• Careful monitoring and notation of cognition, alertness, and basic vital signs should be performed every 30 minutes for the first 4 hours postbaclofen injection and then at least every 2 hours between hours 4 and 8 postinjection.
• Oral intake of food and liquid should not be resumed until the patient is shown to have adequate alertness and cognition and at least 2 hours has passed since the single shot baclofen injection
• Postoperative antibiotics during the course of injection is debatable and is not currently supported by the scientific literature.
Other Adverse Events for Single-Shot Trial
• Bladder changes
• Paralysis
• Death
• Spinal headache
• Epidural hematoma
• Epidural abscess
• Intrathecal hematoma
• Intrathecal abscess
• Discitis
• Injury to adjacent nerve root
• Skin infection including cellulitis and abscess
• Skin erosion
• Skin irritation or allergy to skin preparation
• Anaphylaxis from any medications utilized
• Allergy to hardware or other supplies utilized
• Catheter dislodgement

Full access? Get Clinical Tree






