FIGURE 27.1 Intracranial pressure (ICP)–volume curve. The curve has three parts: A flat part representing good compensatory reserve (A–B), an exponential part representing reduced compensatory reserve (B–C), and a final flat part representing terminal derangement of cerebrovascular responses at high ICP (C–D). (Reproduced from Smith M. Monitoring intracranial pressure in traumatic brain injury. Anesth Analg. 2008;106:240–248, with permission.)
3. In order to appreciate the utility behind ICP monitoring, an understanding of the negative consequences of elevated ICP and its relationship to CPP should to be understood.
a. CPP is dependent on mean arterial pressure (MAP) and ICP as represented by the following equation:
CPP = MAP – ICP
b. According to this relationship, CPP can be reduced from an increase in ICP, a decrease in blood pressure, or a combination of both. As CPP is reduced below the lower threshold of autoregulation (usually below 60 to 70 mm Hg), CBF drops precipitously as the autoregulatory ability is lost (see Chapter 1).
c. Due to the relative inflexibility of the cranial vault, increased ICP causes a critical reduction in CPP and cerebral blood flow (CBF) leading to secondary ischemic injury. If left untreated, sustained elevations in ICP also generate pressure gradients within the cranial cavity that can lead to herniation of brain tissue through the tentorial hiatus or foramen magnum. In the case of foramen magnum herniation, pressure on the brainstem results in a Cushings response (bradycardia, hypertension), and if left untreated, respiratory depression and death. This makes the recognition and treatment of elevated ICP critical [1,5].
CLINICAL PEARL
Increased ICP causes a critical reduction in CPP and cerebral blood flow (CBF) leading to ischemic injury.
4. Several physiologic factors influence ICP. These include arterial carbon dioxide (CO2) reactivity, metabolic coupling with CBF, and blood pressure autoregulation.
a. Carbon dioxide directly affects vascular tone which in turn changes ICP (see Chapter 1). As CO2 tension in the blood increases, the cerebral vasculature vasodilates increasing CBF and hence ICP. Similarly, as the PaCO2 decreases, CBF and ICP do so in a proportional manner. Hyperventilation has historically been utilized to lower ICP but has lost favor in recent years due to a concomitant risk of precipitating cerebral ischemia (see Chapter 1). Evidence suggests that hyperventilation should only be employed in the case of emergent management of intracranial hypertension [10].
b. Decreasing cerebral metabolic oxygen consumption (CMRO2) also has the potential to decrease ICP since the normal brain adjusts arterial blood flow to metabolic demand (see Chapter 1). Although this mechanism is not always preserved, decreasing CMRO2 theoretically reduces CBF [1,11].
c. The effect of blood pressure on ICP is more complex and depends on whether or not CBF autoregulation is preserved. When this mechanism is intact and blood pressure is raised within the autoregulatory range, CBF remains constant due to a reduction in arteriole diameter and an increase in cerebrovascular resistance. However, when cerebral autoregulation is impaired, these same vessels dilate passively and increase ICP by increasing intracerebral blood volume. In addition, the increase in blood pressure is transmitted to the capillary bed worsening intercapillary hydrostatic pressure and worsening edema. Adding to the complexity is the fact that autoregulation may not be simply preserved or absent but rather has varying degrees of autoregulatory capacity [1].
B. Etiology
1
1. TBI is the most common primary cause of elevated ICP. Other etiologies include intracranial hemorrhage, ischemic infarction, and intracranial neoplasms (Table 27.1). A number of mechanisms are responsible for the changes in intracranial volume that subsequently lead to elevation in ICP. In the case of TBI, traumatic hematomas may collect in the intracerebral, subarachnoid, subdural, or extradural spaces creating pressure gradients within the cranium leading to brain shifts. Cerebral edema, hyperemia, and hydrocephalus also contribute to this increase in intracranial volume [1].
Table 27.1 Causes of elevated ICP
2. ICP waveforms (see Figs. 27.2–27.5 below)
a. ICP waveform analysis is complex but can yield valuable insight into the pathophysiology of raised ICP. The normal ICP waveform is a result of small pulsations transmitted from the systemic blood pressure to the intracranial cavity that are superimposed on slower oscillations caused by the respiratory cycle [4]. Pathologic waveforms include A, B, and C types, all described by Lundberg in the 1960s [4]. “A” waves, also known as plateau waves, occur in any number of conditions and reflect an increase in ICP from normal to moderately elevated to gross intracranial hypertension. They occur in patients whose autoregulatory ability is intact and are due to intense vasodilation in response to decreased cerebral perfusion. ICP rises to between 50 and 100 mm Hg and lasts from 5 to 20 minutes. They are always pathologic and should raise concern as their presence may be associated with early signs of brain herniation such as bradycardia and hypertension. Furthermore, they lead to the development of a vicious cycle causing decreases in CPP leading to more “A” waves and further reductions in CPP and eventually irreversible cerebral ischemia [3,5,12,13]. “B” waves occur at a frequency of 0.5 to 2 waves/min with elevations in ICP 20 to 30 mm Hg above baseline. These waves reflect changes in vascular tone that occur when CPP is at the lower limit of pressure autoregulation. “C” waves occur simultaneously with ABP at a frequency of 4 to 8/min with smaller amplitude than “B” waves. They reflect changes in systemic vasomotor tone and have little pathologic significance [5].
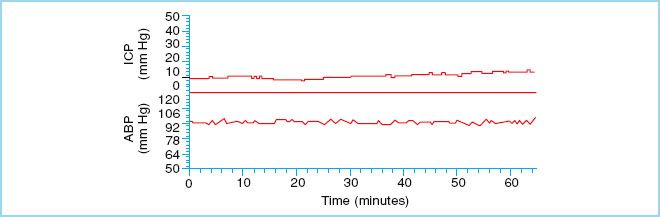
FIGURE 27.2 Normal ICP waveform. Mean arterial blood pressure (ABP) is plotted along the lower panel. (Reproduced from Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiarty. 2004;75(6):813–821, with permission.)
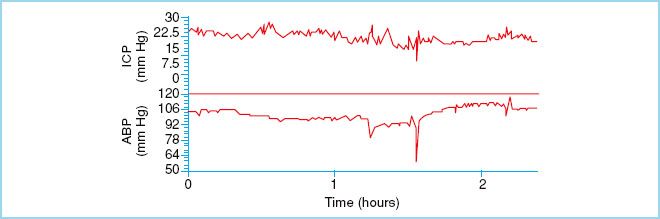
FIGURE 27.3 Represents stable and elevated ICP. This waveform is seen the majority of time in head injury patients. (Reproduced from Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiarty. 2004;75(6):813–821, with permission.)
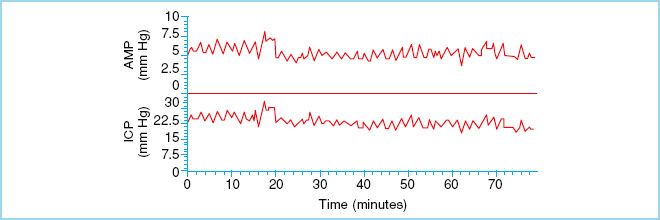
FIGURE 27.4 “B” waves of ICP. These waves reflect changes in vascular tone that occur when CPP is at the lower limit of pressure autoregulation. (Reproduced from Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiarty. 2004;75(6):813–821, with permission.)
FIGURE 27.5 “A” waves, also known as plateau waves, occur in any number of conditions and reflect an increase in ICP from normal to moderately elevated to gross intracranial hypertension. They are always pathologic. (Reproduced from Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiarty. 2004;75(6):813–821, with permission.)
III. Types of ICP Monitoring
A. Overview. Over the years, a variety of devices have been used to monitor ICP with varying degrees of success. Modalities differ with regard to the type of measurement sensor used and the intracranial location chosen. Each offers advantages and disadvantages in terms of ease of insertion, invasiveness, complication rate, and practicality. An ideal device meets various specific requirements including accuracy of absolute measurements (tolerance), constant values over timely measurements (drift), low dependency from previous or consecutive measurements (hysteresis), accuracy of repeated measurements (validity), and accuracy of the absolute value dependent on the magnitude of the value (linearity) [14]. Although most currently available devices are invasive, the development of accurate, noninvasive alternatives is underway.
B. Invasive monitoring
1. Monitor type
2
a. Intraventricular. An intraventricular catheter, also known as ventriculostomy or EVD is considered the gold standard for monitoring ICP and is therefore the method to which all others are compared. A ventriculostomy is an open-ended conduit placed directly into the CSF of the lateral ventricle. Once in place, it is connected to a standard pressure transducer via fluid-filled catheter, and zero referenced at the external auditory meatus. In addition to continuous monitoring of ICP, these devices, with the addition of a 3-way stopcock, can be used to drain CSF for diagnostic and therapeutic purposes. Although this is considered advantageous, it is important to realize that draining CSF and measuring ICP simultaneously leads to inaccurate recordings that underestimate actual ICP. Therefore, performing these two functions separately is conducive to accurate monitoring. These catheters can also be used to administer drugs such as antibiotics and thrombolytic agents, and have the added advantage of periodic external recalibration. Disadvantages include difficult placement, particularly when raised ICP causes compression or shifting of the ventricles leading to bleeding and inaccurate recordings. Fluid leaks, air bubbles, blood clots, and brain tissue can all lead to erroneous ICP readings by interfering with pressure wave conduction from the ventricles to the external transducer. Also, in order to obtain accurate ICP readings, the transducer must be re-zeroed each time the level of the patient’s head is altered. Perhaps the biggest disadvantage to this system is that the ventricular catheter pierces the meninges and brain, potentially leading to serious infection [14]. CSF sepsis is the most serious of these with infection rates ranging from 0% to 45% [15]. This complication leads to increased morbidity and mortality as well as longer hospital stays and higher hospital costs, and can be significantly reduced with careful attention to aseptic technique when placing and managing the catheter [15]. The development of antibiotic-impregnated catheters has further lowered the incidence of infection [16]. As mentioned, intraventricular catheterization remains the gold standard method of ICP monitoring and the initial modality chosen in most instances where ICP monitoring is necessary. However, risks related to infection, hemorrhage, obstruction, and misplacement have led to the development of alternative methods of ICP monitoring.
CLINICAL PEARL
Ventriculostomy is considered the gold standard for monitoring ICP.

Full access? Get Clinical Tree







