KEY POINTS
Interventional radiology (IR) provides a gamut of minimally invasive therapies well suited for the critical care patient population.
The dictum of “smaller, faster, safer, better” is the ideal of minimally invasive image-guided therapy. In the appropriate patient, this type of therapy is invariably better tolerated than more invasive techniques.
Three primary image modalities are used to guide IR procedures: fluoroscopy, computed tomography (CT), and ultrasound (US). Increasingly, hybrid suites are equipped with all three modalities
Appropriate ICU-monitoring devices and support personnel must be available in the IR suite to best serve the critical care population.
Interventional radiology (IR) is a field of medicine devoted to using image-guided minimally invasive techniques to improve patient care. Rather than being unified by an organ system or disease, interventional radiologists are guided by the dictum of “smaller, faster, safer, better” therapy. As such, the interventional radiologist treats patients of all demographics. Commonly, IR procedures are performed instead of traditional open surgical procedures because minimally invasive procedures are often better tolerated with less morbidity and lower mortality. This is particularly important in critical care patients who often have significant comorbidities. The overwhelming majority of procedures offered in the IR suite are performed using conscious sedation, which also tends to limit risks associated with these therapies. As such, it is critical that patients be able to minimally cooperate with interventional radiologists. If patients are combative or unable to lie still, anesthesiologists may be required to assist.
WHERE SHOULD THERAPY BE PERFORMED?
Provided appropriate personnel and monitoring devices are available, as a general rule, the safest and best place to perform an IR procedure is unquestionably in the IR suite. Some very straightforward procedures such as drainage of a large, superficial abscess can be done at bedside but there are significant disadvantages to initiating IR therapy in the ICU. First, the safety and effectiveness of nearly all IR procedures are predicated on high-quality imaging. In many procedures, more than one imaging modality is used in an IR suite to provide the largest margin of safety. For example, when cholecystostomy is performed in the IR suite, the gallbladder is punctured using ultrasound (US) guidance and the remainder of the procedure is completed using fluoroscopic guidance. While it is possible to perform the procedure using only US guidance at bedside, sonographic visualization of needles, wires, dilators, and tubes may be limited, particularly in large patients. Portable fluoroscopy units are typically inadequate because they are awkward, have a small field of view, and provide no meaningful radiation shielding. Second, and more importantly, an interventional radiologist has a limited ability to recognize and treat any complication that occurs during a bedside procedure. Complications that may prove lethal at bedside may be easily handled in an IR suite given the superior imaging and immediate access to specialized catheters and other equipment. One common dilemma involves the patient who needs an IR therapy but is “too unstable” to travel to the IR suite. In our collective experience, as a rule of thumb, patients that are too unstable to travel are usually also too unstable to undergo IR therapy and may poorly tolerate attempts to initiate therapy at bedside. Clearly, there are exceptions and the risks and benefits of any therapy are dictated by local expertise and must be carefully considered and discussed among the ICU team and IR team.
PREPROCEDURAL PREPARATION
It is especially important for critically ill patients to be properly prepared for IR therapy. If patients are obtunded or combative and will be unable to lie still, a consultation with an anesthesiologist is strongly recommended. When possible, coagulopathies should be corrected. When this is not possible, procedures should be delayed or modified. For example, an arterial sheath may be left in place after completion of angiography to be removed later. Heparin should be discontinued at least 2 hours prior to procedures and restarted 6 to 8 hours after completion of procedures as a drip (no bolus). If the patient is allergic to contrast, preprocedural medications should be given whenever possible.
Acceptable guidelines will vary slightly from institution to institution but general guidelines for patients to undergo IR therapy are
International normalized ratio <2.0
Platelets >75,000
Contrast allergy premedication: methylprednisolone 32 mg, 12 and 2 hours prior to contrast administration, diphenhydramine 50 mg, 1 hour before contrast administration (ACR Manual on Contrast Media, 2010)
If the patient has renal insufficiency, this should be discussed with the interventional radiologist because iodinated contrast material is nephrotoxic and should be avoided unless absolutely necessary. In many cases, alternative contrast agents such as carbon dioxide may be used to facilitate therapy. Carbon dioxide enhancement may be used to guide inferior vena cava (IVC) filter insertion, transjugular intrahepatic shunt creation, and to perform diagnostic angiography below the chest. In the past, gadolinium was used in patients with renal insufficiency. Currently, due to the associated risk of nephrogenic systemic sclerosis, this practice has been discontinued.
PATIENT MONITORING IN IR
While the level of monitoring equipment and specialized staff varies from institution to institution, in general, all state-of-the-art IR suites are outfitted with basic patient monitoring equipment including electrocardiography, noninvasive pulse oximetry, and automated blood pressure monitoring. Wall suction and oxygen are also ubiquitous and newer rooms can monitor end-tidal carbon dioxide, which can detect respiratory depression sooner than pulse oximetry. Every IR suite is staffed with at least one technologist and one nurse in addition to the physician(s) providing therapy. In our hospital, the majority of our IR nurses have ICU experience; during procedures, they administer sedation (typically midazolam and fentanyl) and monitor the patient.
Critically ill patients from the ICU should be accompanied to IR with equipment and staff that can handle any additional life supportive measures. In the authors’ opinion, patients are best served if a physician from the ICU also accompanies the patient to the IR suite. In a very practical sense, it is impossible for an interventional radiologist to both competently perform an image-guided procedure while simultaneously directing supportive therapy in a critically ill patient unfamiliar to him or her. Optimal patient care dictates constant communication and close cooperation between the ICU and IR services throughout this process.
PERCUTANEOUS ABSCESS DRAINAGE
KEY POINTS
Percutaneous abscess drainage is the treatment of choice for infected, well-defined fluid collections.
Percutaneous drainage is the treatment of choice for abscesses and other fluid collections such as urinomas and bilomas. Compared with surgical exploration, percutaneous approaches are less invasive and associated with decreased mortality.1 In some instances, percutaneous approaches are less costly. Percutaneous drainage is particularly favored in critically ill patients as they are often not surgical candidates.
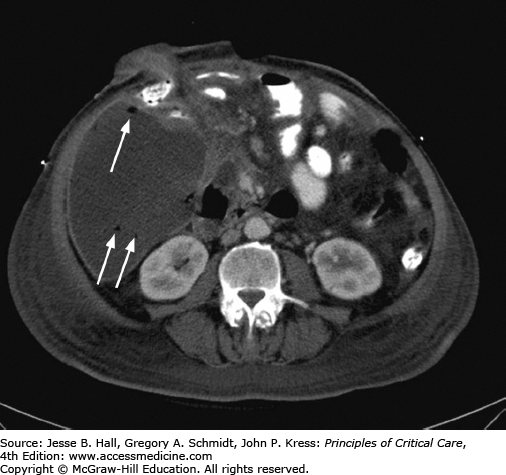
When an abscess is suspected in an ICU patient, cross-sectional imaging is typically performed. CT scanning is preferred over sonography. If possible, oral and intravenous contrast should be administered. Enteric contrast aids in differentiation between an abscess and adjacent bowel loops. CT allows superior visualization of adjacent organs and better planning of the access route. US is operator dependent and limited by patient body habitus, dressings, and the inability to penetrate gaseous interfaces. However, sonography is superior at detection of septations and loculations within a collection and may be used in conjunction with radiography for pleural space collections. US may also be sufficient for detection of solid organ abscesses. Once a collection is identified, it is crucial to realize that an abscess (or biloma, urinoma, lymphocele, hematoma, etc) cannot be diagnosed based on the imaging appearance alone. However, a thick enhancing wall and gas within the collection suggest the diagnosis (Fig. 30-1).
The size of the collection is also important. It is usually difficult or impossible to insert a drainage catheter into a collection, which is only 1 or 2 cm in diameter, and it should be remembered that a spherical collection 2 cm in diameter contains only a little more than 4 cc of fluid. With small collections we often perform a simple fluid aspiration with a needle. Once a collection is 3 cm or greater in diameter, a pigtail drainage catheter can usually be secured.
The main relative contraindication to consider is coagulopathy. We routinely obtain coagulation parameters including platelet count, prothrombin time (PT), international normalized ratio (INR), and activated partial thromboplastin time (aPTT) and correct any underlying coagulopathy prior to the procedure. Antiplatelet medications are ideally held for at least 3 days, though this is often not feasible in emergent situations. Heparin is typically discontinued for at least 2 hours.
Appropriate antibiotics should be initiated prior to the procedure because manipulation of the abscess can result in bacteremia and spread of contents into sterile cavities. Conscious sedation is preferred, though in select circumstances, general anesthesia may be necessary. In some patients, the procedure can be performed with local anesthetic only. A thorough review of imaging studies will determine the safest access route. The best route is usually the shortest and straightest pathway. Ideally, the catheter is placed in a convenient location for ongoing care. In solid organ collections, a small amount of normal parenchyma is traversed to aid fixation and mitigate against peritoneal or retroperitoneal spillage. Large, superficial collections can often be drained sonographically with fluoroscopic guidance. US is readily available, typically has a shorter procedure time than CT, and provides the best visualization of direct needle advancement and adjacent vascular structures. Other drainage procedures require CT guidance to confirm appropriate catheter positioning. While most collections are accessible percutaneously, deep pelvic abscesses pose unique problems. The pelvic bones, bladder, bowel loops, and rich pelvic vasculature pose many obstacles to a direct percutaneous path. Additionally, percutaneous transgluteal drainage is often painful (especially when above the level of the piriformis muscle) and risks injuring the sciatic nerve and sacral plexus. In these cases, US-guided transrectal or transvaginal drainage may be necessary. These are surprisingly well tolerated with the most frequent complication being catheter dislodgement.2
If the nature of the collection is uncertain, diagnostic fluid aspiration with a 20- or 22-gauge needle can be performed first. If the sample obtained is pus, a drainage catheter can be placed.
Large collections can be drained by a one-stick, trocar technique. The drainage catheter is preloaded on a sharp stylet. Analogous to placement of a peripheral intravenous line, once the collection is entered, the catheter is advanced over the needle into the collection. The stylet is then removed and the contents are aspirated. This technique is especially useful during endocavitary approaches. Most collections, however, are accessed using an over-the-wire Seldinger technique. This allows verification of successful access prior to the creation of a large bore tract. Unless the collection is large, we typically enter the collection with a 22-gauge needle and coil an 0.018-in guide wire in the collection. Over this microwire, a coaxial 5- or 6-French sheath/dilator assembly is then advanced into the collection, allowing placement of a 0.035-in wire. Over the larger wire, a locking loop catheter with an inner metal or plastic stiffener can then be advanced. It is usually necessary to dilate the soft tissue tract with fascial dilators prior to final placement of the drain. Disadvantages of the Seldinger technique include the potential for loss of access and cross-contamination during exchanges.
A wide array of drainage catheters is available. Locking pigtail catheters are most commonly used. Most collections can be adequately drained with 6- to 12-French pigtail drains, though if the collection contains highly viscous fluid or extensive debris, a larger drain may be necessary. Contrast can be injected into the drain to better define the collection and visualize fistulas. Though the pigtail helps secure the tube, skin sutures and adhesive locking dressings add an extra measure of security against accidental tube dislodgement. At the time of placement, we strive to completely aspirate the collection. The catheter is then placed to gravity or bulb suction and output is documented. With thick complex collections, saline or fibrinolytic irrigation can be used to facilitate drainage.3
Close follow-up after catheter placement is essential to ensure adequate drainage and detect delayed complications. Normally, the catheter output will gradually taper off. Most drainage catheters are kept in place for 3 to 7 days. If output has diminished but the patient has not clinically improved, the catheter should be flushed with a small amount of saline to ensure that it is not clogged. If the catheter is not clogged but appropriately positioned, catheter exchange or upsize and/or fibrinolytic therapy may be necessary. If large volume output persists, an enteric fistula may be present. We usually use defervescence, resolution of leukocytosis, and catheter output of <10 cc/24 hours as indicators of success and will consider catheter removal without repeated imaging if these conditions are met. If not, repeat imaging should be performed.
Technical success exceeds 90% and is immediately apparent. Clinical success rates depend on abscess location and complexity, underlying immune status, and subsequent fistula formation. Overall, however, clinical success ranges from 70% to 90%. Overall complication rates for percutaneous abscess drainage range from 10% to 15%, with serious complications accounting for less than 5% of cases.4 Infectious complications may be encountered during primary catheter placement, when spread of abscess contents or bacteremia can occur. As a result of prolonged catheterization, skin-site infections can occur. Hemorrhage can occur from venous or arterial transection or from the development of pseudoaneurysms or vascular fistulas. Minor bleeding is usually self-limited and managed conservatively. In these cases, temporary tube capping, upsizing, or repositioning may help tamponade the bleeding. When more substantial hemorrhage is suspected, angiography and surgical consultation are usually necessary. Inadvertent catheterization or puncture of adjacent organs or bowel may also occur.
PERCUTANEOUS NEPHROSTOMY
KEY POINTS
Percutaneous nephrostomy should be performed for urinary diversion when retrograde ureteral stenting fails or is contraindicated.
Hematuria is very common after nephrostomy and should slowly regress over several days. Persistent hematuria suggests vascular injury.
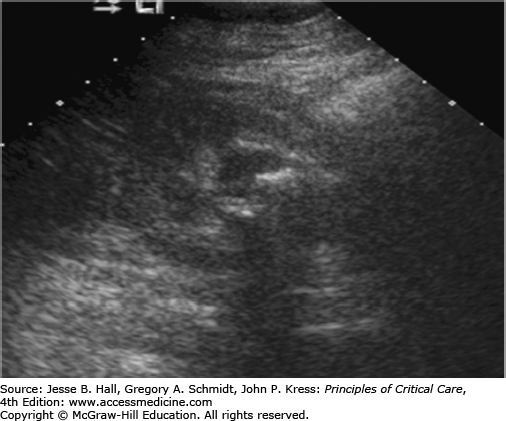
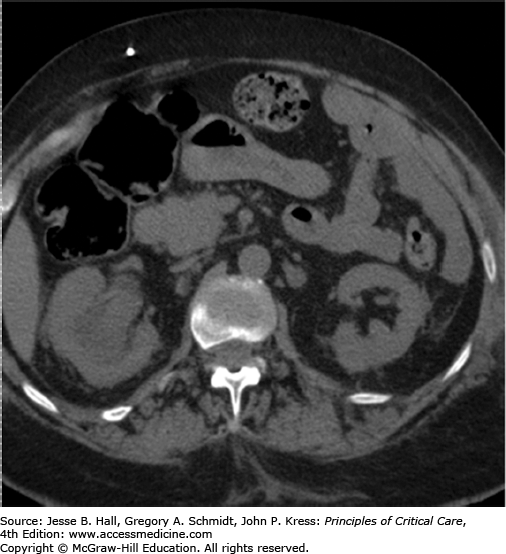
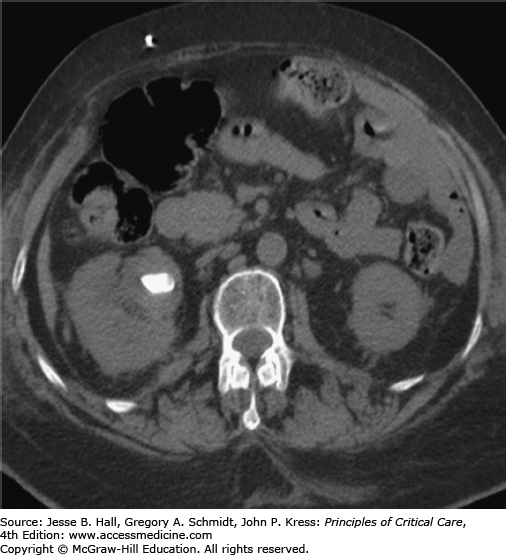
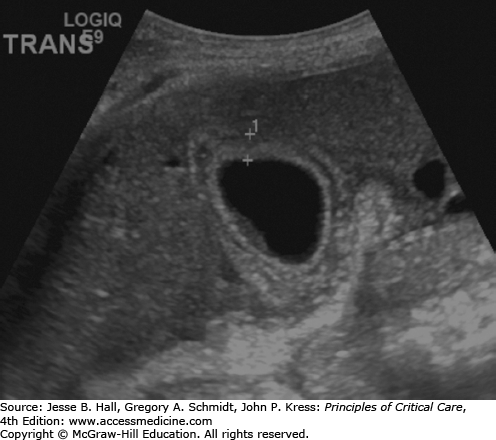
In the ICU setting, fever associated with urological obstruction carries a high risk of mortality caused by urosepsis, and percutaneous drainage of the collecting system is a well-established, first-line option for emergent decompression. Most commonly, percutaneous nephrostomy (PCN) and percutaneous nephroureterostomy (PCNU) are performed to treat acute ureteral obstruction caused by urolithiasis, but they are also indicated for symptomatic malignant obstruction as well as for urinary diversion from fistulas involving the urinary tract and ruptures complicating other modalities such as ureteroscopy. The diagnosis of urinary tract obstruction is most often made by US or CT. In the ICU setting, US has the advantage of portability and provides excellent evaluation of the proximal collecting system for stones and obstruction (Fig. 30-2). CT may further detect evidence of life-threatening pyonephrosis (Figs. 30-3 and 30-4) or emphysematous pyelonephritis, and provides more complete visualization of the renal collecting system when required.
In most institutions, the septic patient with a urinary tract acutely obstructed by urolithiasis is treated preferentially with percutaneous drainage. Ureteroscopic procedures to bypass or remove stones are deferred pending interval resolution of hemodynamic instability, fever, and leukocytosis. However, published literature comparing percutaneous nephrostomy and retrograde ureteral stent placement for the treatment of acute septic obstruction caused by urolithiasis has demonstrated similar technical and clinical success rates,5,6 and both procedures remain viable, first-line options for this indication. The presence of thrombocytopenia associated with sepsis makes percutaneous access less optimal in many cases, and severe, uncorrectable coagulopathy is the main, relative contraindication to PCN.7
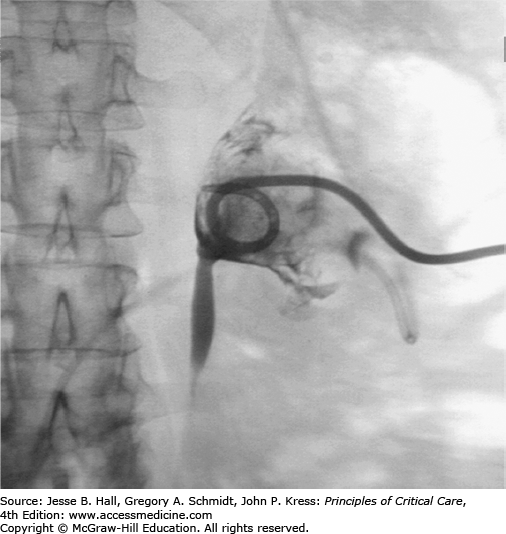
PCN and PCNU are best performed in the IR suite using a combination of US and fluoroscopic guidance with the patient in the prone or semiprone position under moderate sedation. Preprocedural antibiotics are administered unless broad-spectrum antibiotic coverage has been initiated prior to referral to radiology. Using Seldinger technique, a 21- or 22-gauge needle is advanced through a posterior calyx into the renal pelvis under US guidance, urine is aspirated to verify access, contrast is gingerly injected, a coaxial dilator is used to convert from microwire to standard wire access, and fluoroscopic guidance is used during tract dilatation and catheter placement. Performance of a diagnostic nephrostogram involves intraluminal distension by contrast material with the risk of symptom exacerbation, and is often deferred pending resolution of fever and leukocytosis. The retention mechanism of a self-retaining catheter (typically locking-loop type) is secured within the renal pelvis (Fig. 30-5), and the catheter is placed to gravity drainage. Urine is sent for culture and sensitivity testing. When PCNU is required, manipulation of a 5-French catheter and guide wire into the urinary bladder precedes tract dilatation and internal-external PCNU placement.
Patients are carefully observed overnight for evidence of bleeding or exacerbation of systemic infection following percutaneous drainage of the kidney. Fluid input and catheter output are recorded every shift, and broad-spectrum antibiotics are administered until coverage needs are dictated by culture and sensitivity results. Catheters typically remain in place until fever and leukocytosis have resolved, the cause of the obstruction has been treated, and adequate time has passed for healing and tract formation to minimize the risk of bleeding—typically 1 to 2 weeks. In cases involving urolithiasis, stones may pass spontaneously, require percutaneous nephrolithotomy by basket or snare in the IR suite, or require extracorporeal shock wave lithotripsy by urologists. If prolonged PCN or PCNU is required, fluoroscopically guided catheter exchange is recommended every 4 to 6 weeks. PCNU catheters can be capped for patient comfort if the patient remains asymptomatic. In many cases involving neoplastic, fibrotic, inflammatory, or iatrogenic obstruction, prolonged internal drainage is preferred to external drainage for patient comfort, and the PCN or PCNU can be converted to internal double J ureteral stents in the IR suite. Internal stents require routine cystoscopically guided changes every 3 months by urology.
Management of the PCN catheter is typically a combined effort by the ICU team and rounding IR staff. Typically, the collected urine progresses from blood tinged to clear over 2 to 3 days. Severe bleeding at the time of placement may respond to capping the catheter for a few hours to create a tamponade effect. Delayed onset of bleeding or persistent low-grade bleeding is typically caused by venous injury and addressed by repositioning or upsizing the catheter under fluoroscopic guidance. Leakage of urine around the skin entry site, or lack of timely resolution of clinical symptoms, may indicate tube dislodgment or obstruction, and evaluation under fluoroscopy or by cross-sectional imaging may be indicated. Inadvertent retraction of the tube can be treated with exchange, if any access into the kidney has been maintained, or complete replacement if access has been lost. Depending on severity, skin-site infections can be addressed with antibiotics and fluoroscopic catheter evaluation, or in more severe cases, placement of a new catheter at a different site or internalization to a double J stent if this option exists.
PCN is successful in cases of dilated, obstructed collecting systems in 98% to 99% of cases in published literature spanning decades.7 Lower success rates are encountered in the absence of pelvocaliectasis and in the presence of complex staghorn calculi. Major and minor complications occur in approximately 10% of patients. The most common major complication is sepsis or exacerbation of systemic infection, most commonly associated with the presence of pyonephrosis.8 Overdistension of the renal collecting system should be strictly avoided in these patients. Pleural complications such as pneumothorax, hemothorax, or empyema reportedly occur in 9% to 12% of patients undergoing PCN via an intercostal window.9 Other reported major complications are less common and include hemorrhage and colon transgression.
PERCUTANEOUS CHOLECYSTOSTOMY
KEY POINTS
Indications for percutaneous cholecystostomy appear to be increasing.
Cholecystostomy catheters must remain in place long enough (usually >2 weeks) for a track to mature prior to manipulation or removal.
Patients with acute cholecystitis in the ICU are often at high risk for morbidity and mortality associated with surgical treatments such as open or laparoscopic cholecystectomy. Percutaneous cholecystostomy (PC) has been established as a definitive treatment, a bridge to surgery, or a means toward adjunctive, minimally invasive therapies, depending on patient presentation.10,11
In the case of acute calculous cholecystitis, surgical cholecystectomy remains the first-line therapy in surgical candidates. In low-risk patients, published periprocedural mortality rates of both open and laparoscopic cholecystectomy are typically below 1%.12 In patients deemed too unstable to undergo surgery and/or general anesthesia, PC serves as bridge to more elective surgery or, in permanently high-risk, comorbid patients, a bridge to adjunctive therapies such as gallbladder ablation, stone dissolution, shock-wave lithotripsy, and/or basket extraction.13-16 Adjunctive techniques for stone removal have been associated with a high rate of gallstone recurrence in retrospective studies—10% to 30% per year with a symptomatic recurrence rate of approximately 6% to 18% per year.17 Therefore, most high-risk patients undergo eventual surgical cholecystectomy, and poor candidates may require permanent cholecystostomy.
In the case of acute acalculous cholecystitis, the drainage catheter can be removed after resolution in most cases, without the need for elective interval cholecystectomy, since the risk of recurrence is likely to be low (<10%) based on retrospective studies.15 Predisposing conditions include diabetes, malignancy, burn injury, recent surgery, recent trauma, cardiac disease, positive pressure ventilation, and total parenteral nutrition. Establishing the diagnosis remains a clinical challenge since the accuracy of US (Fig. 30-6) is approximately 50% to 60%, and the false-positive rate of nuclear medicine hepatobiliary scans is approximately 25% to 30%, caused by factors such as liver dysfunction, sepsis, fasting, and prolonged total parenteral nutrition. In many cases, a high clinical suspicion by the critical care team leads to PC in the setting of soft radiological support. Patients with true acute acalculous cholecystitis typically show a quick and marked clinical response to PC.
PC is best performed in the IR suite using US and fluoroscopic guidance under moderate sedation, but can be done in select cases at the bedside using only portable US guidance. Difficult or complicated cases may require CT guidance.
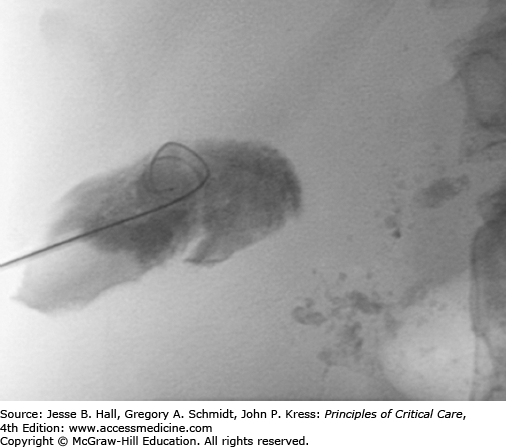
Patients are typically referred to interventional radiology after initiation of broad-spectrum antibiotic coverage; otherwise, preprocedural antibiotics are administered. Authors have described successful applications of both Seldinger and trocar techniques. The advantage of Seldinger technique is verification of creation of an access tract to the gallbladder using a low-gauge needle prior to dilation and placement of a drainage catheter. The advantage of trocar technique is placement of a drainage catheter in a single step, without the potential for bile leakage associated with serial tract dilatation. In Seldinger technique, a 21- or 22-gauge needle is advanced into the gallbladder under US guidance, bile is aspirated, contrast is gingerly injected (Fig. 30-7), a coaxial dilator is used to convert from microwire to standard wire access, and fluoroscopic guidance is used for tract dilatation and catheter placement. In trocar technique, a small-bore catheter fitted over a stiffening cannula and sharp stylet is advanced as a unit under US guidance, and the catheter is advanced off the cannula directly into the gallbladder. Most radiologists place self-retaining, locking loop catheters. While there is likely no difference in the incidence of peritonitis after transperitoneal versus transhepatic placement, transhepatic placement may improve stability during and after placement and is favored by some radiologists. Gram stain and culture results of the bile are not sensitive (30%-50%) but may aid in determining specific antibiotic therapy when positive.
Cholecystostomy catheters are drained to gravity bag, and output is monitored every shift. If the cystic duct is indeed obstructed, low volumes of clear mucus (50-70 mL) are expected daily. Larger volumes of biliary drainage indicate cystic duct patency, and very large volumes (>1 L) indicate obstruction of the distal common bile duct and patency of the cystic duct, usually the result of stone migration. Management of the cholecystostomy catheter is typically a combined effort by the ICU team and IR staff. New onset bleeding, leakage of bile around the skin entry site, or lack of timely resolution of clinical symptoms may indicate tube dislodgment or obstruction, and evaluation under fluoroscopy or by cross-sectional imaging may be indicated.
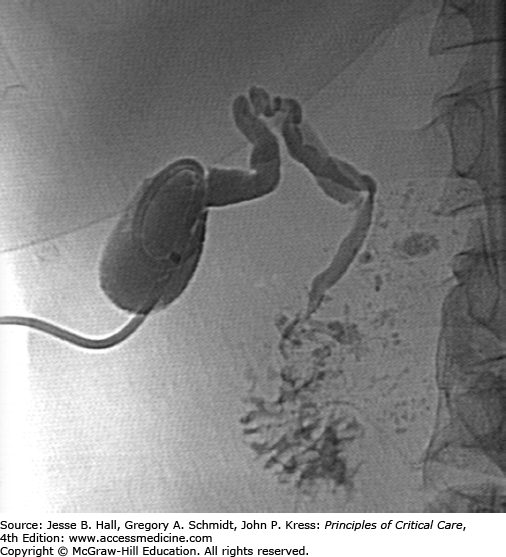
The need for prolonged catheterization should be managed with fluoroscopically guided catheter changes every 4 to 6 weeks. After clinical resolution, patients with acalculous cholecystitis may undergo contrast injection under fluoroscopy. The criteria for catheter removal include the absence of gallstones, patency of the cystic and common bile ducts, free spillage of contrast into the duodenum (Fig. 30-8), and the verification of a mature tract by over-the-wire contrast injection, typically present at 4 to 6 weeks (Fig. 30-9). Patients with calculous cholecystitis face the options of surgical cholecystectomy, adjunctive therapies described above, or permanent cholecystostomy.
FIGURE 30-9
Injection of the cholecystostomy tract with a wire maintaining access in the gallbladder demonstrates an intact tract without spillage of contrast into the peritoneum. This finding coupled with the findings in Figure 30-3 represent the criteria for safe catheter removal. The drain was removed without complications.
Technical success exceeds 95%.19 Clinical success is complicated by the absence of true cholecystitis in many cases, but is approximately 60% for patients with suggestive US findings.20 Major periprocedural complications occur in less than 5% in most published series and include sepsis, hemorrhage, abscess, peritonitis, transgression of intervening structures such as the colon, and death.11 Major postprocedural complications include inadvertent catheter dislodgment or removal, resulting in repeat PC, surgery, or death (<1%).
BRONCHIAL ARTERY EMBOLIZATION
KEY POINTS
Most clinically significant episodes of hemoptysis are caused by bleeding from the bronchial arteries.
Chronic pulmonary diseases (eg, cystic fibrosis, tuberculosis) are the most common underlying disorders predisposing to life-threatening hemoptysis.
Bronchial artery embolization is an effective and safe treatment of massive hemoptysis.
Massive hemoptysis, defined as bleeding greater than 300 mL/24 hours, carries a mortality rate of up to 85% in patients treated by conservative means.21 Recurrent bouts of moderate hemorrhage are also life threatening. Death is usually due to asphyxiation rather than exsanguination or hemorrhagic shock. Surgical resection can be curative for focal disease, but it carries a high mortality rate in the setting of acute hemorrhage. Bronchial artery embolization has proven to be an effective and safe treatment.22,23
Massive hemoptysis typically occurs in the setting of chronic inflammatory lung disease. In 90% of patients, bleeding predominantly arises from the bronchial artery. Tuberculosis, sarcoidosis, and cystic fibrosis are the most common etiologies. Nonbronchial systemic arteries recruited to diseased lung account for 5% of cases. Only approximately 5% of patients with severe hemoptysis have significant bleeding from the pulmonary arterial circulation, however, iatrogenic injury of the pulmonary artery after pulmonary artery catheter insertion should always be considered in the critical care setting.
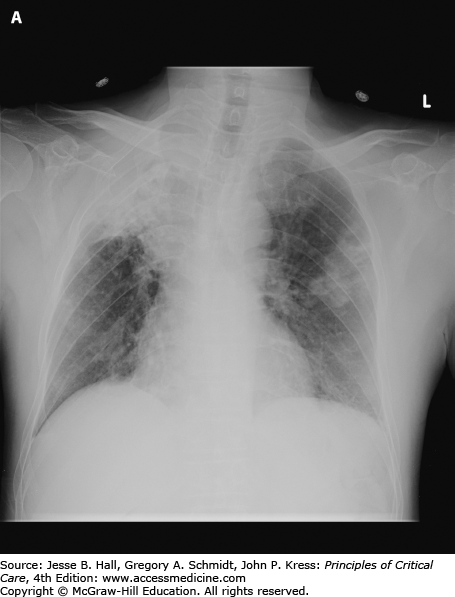
It is imperative that the source of bleeding be localized to the lower airways or upper airway. Upper gastrointestinal hemorrhage can occasionally be confused with bronchopulmonary sources. Anticoagulation and antiplatelet medications should immediately be discontinued and any abnormal coagulation parameters should be corrected. A chest radiograph may help localize the site of bleeding (Fig. 30-10A). CT of the chest is useful for showing areas of bronchiectasis delineating the bronchial artery anatomy, greatly aiding future angiography. Nonbronchial systemic arterial supply can also be evaluated. Bronchoscopy is generally useful when performed early in the patient’s management, though can be limited with severe bleeding. Endotracheal intubation, often with a double lumen tube to protect the contralateral lung, may be necessary. Bronchial balloon occlusion catheters, iced saline lavage, topical medications, laser therapy, and electrocautery can also be helpful in selected cases.
FIGURE 30-10
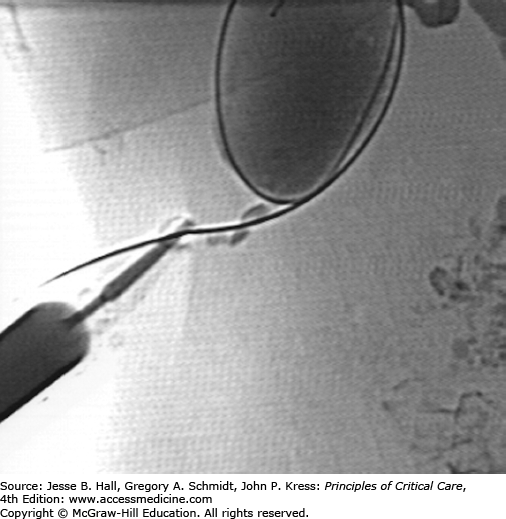
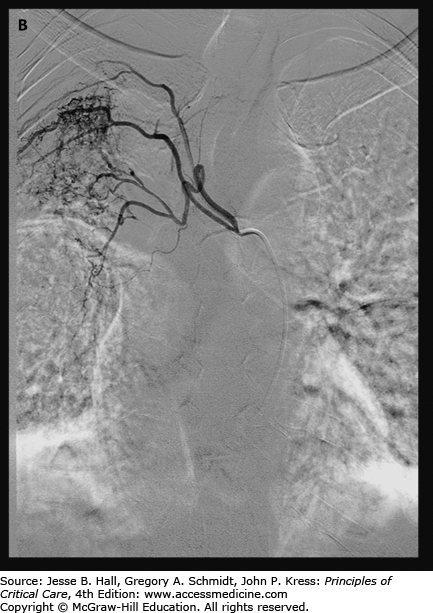
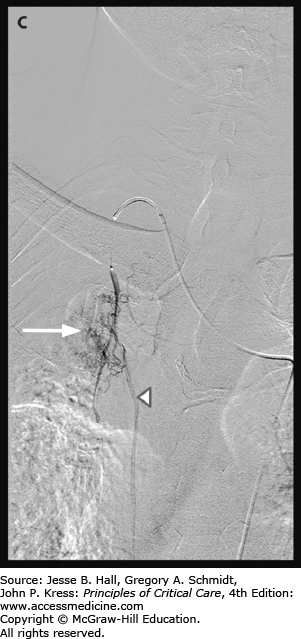
A. Chest radiograph demonstrates multifocal consolidation and bronchiectasis, worst in the right upper lobe. The patient had multidrug resistant TB and recurrent massive hemoptysis. B. Angiography demonstrates an intercostobronchial trunk. The bronchial artery is tortuous and hypertrophied with dense parenchymal blush. C. Angiography of the right internal mammary artery (arrowhead) shows pulmonary parenchymal blush (arrow) contributing to the hemorrhage.
Bronchial artery angiography and embolization is typically performed via a common femoral artery approach. Bronchial arterial anatomy is variable, though typically the origins arise near T5 or T6. Most commonly, one or two bronchial arteries are present on each side. A thoracic aortogram can be performed to better define the anatomy. On the right, an intercostobronchial trunk is common. Special consideration is given to potential embolization of the anterior spinal artery, the dominant arterial supply to the spinal cord. Given that embolization of the cord may cause permanent injury, the procedure may need to be aborted. Alternatively, if a microcatheter can be advanced distally beyond any spinal arteries, embolization may be pursued.
Once the bronchial artery is catheterized, selective angiography is performed. Often, a 3-French microcatheter is used to obtain more secure access into the bronchial artery. Frank extravasation is usually not seen. However, hypertrophy, hypervascularity, and aneurysms are commonly visualized (Fig. 30-10B). When the artery is abnormal, embolization is performed. The goal of treatment is to provide effective embolization without affecting the capillary bed of the bronchus. A wide variety of agents have been used successfully though medium-sized particles are most commonly used. The angiogram should be carefully evaluated for potential bronchial artery to pulmonary vein shunts. Smaller particles could traverse shunts and then enter the systemic arterial circulation. If these shunts are present, larger particles or coils may be necessary to close the shunt prior to proceeding with the embolization. If the bronchial arteries appear normal or do not entirely account for the hemoptysis, a search of nonbronchial systemic collateral supply is performed (Fig. 30-10C).

Full access? Get Clinical Tree