Chapter 96 Infectious Syndromes in the Pediatric Intensive Care Unit
The pediatric critical care unit is the epicenter of severe infection in most children’s hospitals. Many of these infectious processes are nosocomial and are associated with either patient (e.g., immunocompromised) or procedural issues. Other infectious processes affect specific organs (bronchiolitis and meningitis). These specific infections and the general principles of sepsis management are described in Chapters 29, 47, 65, and 103. In this chapter, the authors describe some of the bacterial, viral, and other infections that may precipitate a systemic response and multiorgan involvement requiring intensive care unit admission. Salient and identifying features are highlighted and, where appropriate, specific comments are provided regarding therapeutic strategies.
Meningococcus
Etiology and Epidemiology
Neisseria meningitidis is a gram-negative diplococcus that is a common cause of bacterial meningitis. It also is responsible for 500,000 cases of a severe sepsis syndrome reported worldwide annually. It is estimated that 1.5 cases per 100,000 persons occur, with 1077 cases reported in 2007 in the United States. The case fatality rate is 10% to 14%.1,2 Of the 13 serogroups, strains A, B, C, Y, and W-135 are implicated most often.3 Most cases are isolated or sporadic, with less than 5% associated with outbreaks.4,5 In the United States, serogroup B accounts for 30% to 70% of sporadic cases, whereas serogroup C is much less common but is often associated with small outbreaks.6–8 Serogroup A is a common cause of cyclic epidemics in Sub-Saharan Africa (“meningitis belt”) and Asia, with increasing outbreaks of W-135 notably in Saudi Arabia in 2001 and in Burkina Faso in 2002.3,9 There were an estimated 700,000 cases in Africa over the past 10 years with an estimated 10% mortality rate.10
The disease most often occurs in children younger than 5 years, with the peak attack rate between the ages of 3 and 5 months. Children with complement C5-9 deficiency and asplenia are at increased risk.3–511 Asymptomatic carriage state has been recognized, and pathogenesis is thought to begin on the nasopharyngeal surface.12 Patients are considered capable of transmitting the organism for up to 24 hours after initiation of treatment.2,5,12
Clinical Presentation
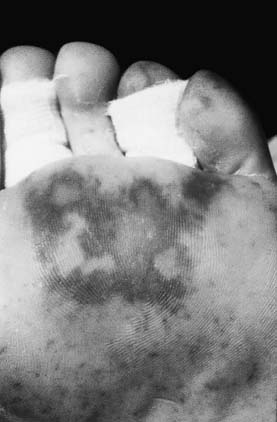
Most invasive meningococcal infections present as meningitis, approximately 10% present as sepsis alone and about 40% present as both systemic and central nervous system (CNS) disease.3,11,13 Since the introduction of the H. influenzae vaccine, N. meningitidis has become the second most common cause of bacterial meningitis in North America after pneumococcus.14 With meningitis alone, the classic signs of headache and meningismus are present but their severity can be variable. Onset of septicemia often is abrupt with fever, chills, malaise, and rash. Rash is the hallmark sign presenting in up to 80% of cases.6,15 Although petechial rash appearing in clusters at pressure sites is classic, urticarial or maculopapular rashes may also be present (Figure 96-1).5 The shock state is mediated by endotoxin (lipopolysaccharide) release with subsequent complement activation and release of numerous inflammatory mediators.12,16,17 Subsequently, rapid endothelial cell injury occurs, leading to capillary leakage, microvascular thrombosis, refractory peripheral vasoconstriction, and acute myocardial failure.16 Septicemia can rapidly progress within hours to disseminated intravascular coagulopathy (DIC), shock, and death. Invasive disease can be complicated by an immune-mediated arthritis, pancarditis, endophthalmitis, pneumonia, and adrenal insufficiency from Waterhouse-Friderichsen syndrome.12,18
Diagnosis
Because of the rapid progression of septicemia and the variability of markers of infection such as white blood cell count and C-reactive protein, clinical diagnosis is imperative.15 Clinical suspicion should be based on the characteristic rash and symptoms and signs of hypoperfusion, such as tachycardia, cool peripheral extremities, decreased urine output, and altered mental state. Aggressive treatment should be instituted prior to the onset of overt hypotension, that usually occurs before culture results are available.19,20
Confirmation may be difficult, particularly when patients have received early antibiotics. Positive cerebrospinal fluid (CSF) and blood cultures range from 50% to 80% in various series.21 Nevertheless, cultures from blood and CSF are indicated. Lumbar puncture should be delayed if evidence of increased intracranial pressure, coagulopathy, or cardiovascular instability is present. Cultures from petechial scrapings and other body fluids such as synovial fluid can be helpful. Because N. meningitidis may be a part of normal flora, nasopharyngeal cultures are not helpful.12 Latex agglutination is helpful for CSF but unreliable for urine and blood specimens. Polymerase chain reaction (PCR) for bacterial protein is being used in various centers, with sensitivity and specificity near 90%.22,23
Management
Conventional Therapy
Experience and epidemiologic data support rapid early intervention in the peripheral center and aggressive treatment in a tertiary care pediatric intensive care unit.19,20,24 Rapid administration of antibiotics prior to transfer has been shown to improve prognosis.25–27 Ceftriaxone 100 mg/kg/day divided once or twice daily is recommended as initial empiric therapy. After N. meningitidis is confirmed, then penicillin G 500,000 U/kg/day divided in six doses can be used based on sensitivities. Chloramphenicol is an alternative if the patient is allergic to penicillin.5,12
Significant capillary leakage is the hallmark feature in meningococcal sepsis. Thus the prominent problem faced in the early stages is maintenance of adequate circulating volume. Early and aggressive volume resuscitation is vital and has been shown to improve outcome.28,29 Normal saline or 5% albumin solutions have been the standard fluids; no evidence indicates that other colloids, such as blood, change outcome. Ongoing need for continued volume resuscitation should be based on clinical signs of shock and may be as high as twice the child’s circulating volume (e.g., 120 mL/kg).11,19,30 Myocardial depression should be assumed and inotropes such as dopamine should be used early concomitantly with fluid to ensure adequate cardiac output. Epinephrine, norepinephrine, or vasopressin may be necessary. However, the need for high-dose α-adrenergic agents and vasopressin to maintain blood pressure may need to be balanced against the risk for distal extremity ischemia and necrosis. To ensure appropriate and rapid volume resuscitation and inotropic delivery, central venous access should be established as soon as meningococcal sepsis is suspected; however, volume resuscitation should not be delayed and should be started with peripheral or intraosseous access if central access is not yet available.30,31
Respiratory support often is necessary when fluid requirements greater than 40 mL/kg are required. Even if the patient is alert and oriented, early intubation and ventilation is beneficial for resuscitation and transport when meningococcus is suspected in order to ensure adequate oxygenation, reduce the patient’s work of breathing, decrease metabolic demands, and maintain stability for transport. More importantly, there is a significant risk of pulmonary edema after aggressive fluid resuscitation in the face of myocardial depression.31
Numerous metabolic derangements often present because of cellular fluid shifts. Abnormal potassium, calcium, and magnesium concentrations can affect myocardial function and should be monitored and managed aggressively. Hypoglycemia is a common finding, and accordingly serum glucose concentration should be monitored closely. Metabolic acidosis is common and usually corrects with adequate perfusion and lactate clearance. Replacement of bicarbonate is reserved for pH less than 7.2 assuming adequate ventilation is present. 28,30,31
DIC is common because of factor loss from capillary leak and clotting factor consumption. Derangements are treated with fresh-frozen plasma and cryoprecipitate as needed to prevent life-threatening hemorrhage. This process is associated with anemia and thrombocytopenia, for which packed red blood cells and platelet transfusions may be necessary, especially if spontaneous bleeding from mucous membranes and venipuncture sites occurs.31
Plastic surgical interventions may be necessary for amputations and skin grafting. Consultation with other services may be necessary for renal failure, secondary infections, and neurologic complications.20,31
Novel Therapy
The role of steroid therapy remains unclear. Exogenous steroids in the septic setting have been thought to enhance upregulation of adrenergic receptors and improve the response to catecholamines, such as exogenous inotropes. No evidence supports the routine use of steroids in meningococcal sepsis specifically but it is indicated in undiagnosed bacterial meningitis.31 Adrenal replacement doses of hydrocortisone 1 to 2 mg/kg have been suggested for fluid and vasopressor recalcitrant septic shock.28,30,32
Immunotherapy, such as antiserum to Escherichia coli, that was thought to halt the immune cascade if given early enough, has not been shown to be beneficial in several trials.33 Likewise, antiendotoxin HA-1A, a human monoclonal immunoglobulin (Ig)M antibody, had no significant benefit.34 The overall limitation of antiendotoxin therapy is that it must be given very early in the disease process in order to halt the inflammatory response. An alternative to antiendotoxins may be bacteriocidal/permeability increasing proteins (BPIs). BPIs are found in neutrophils and neutralize endotoxins after their release. The results of a large multicenter trial were promising but underpowered.35 Anticytokine therapy such as interleukin-1 receptor antagonists and anti–tumor necrosis factor antibodies have been studied for sepsis but not specifically for meningococcus. Plasmapheresis to remove cytokines and other mediators are being studied, but no difference in plasma concentrations or overall outcomes has been reported. Hemofiltration and plasma exchange to remove inflammatory mediators have been performed safely. However, the results are mixed, so they are not currently standard therapy.36
As mentioned, several hemostatic abnormalities are related to meningococcal disease, and it is postulated they are key to pathogenesis and severity of disease. Potential therapies to combat DIC include antithrombin-III (AT-III), tissue plasminogen activator (tPA), activated protein C, and heparin, as reviewed by Leclerc et al.32 Case reports are encouraging, but adequate trials are pending. AT-III infusion may promote return of peripheral perfusion and salvage of limbs.37 Tissue plasminogen activator was associated with an improvement in shock and a decrease in amputations.38 In the largest pediatric sepsis interventional trial (RESOLVE) conducted to date, activated protein C was not superior to placebo in terms of shortening duration of organ dysfunction or decreasing mortality. Similarly, adjunctive treatment with activated protein C does not prevent other morbidity, such as significant loss of limbs and was associated with neonatal bleeding.39,40 Many case reports have supported heparin infusions, but the benefits have not been reproduced in controlled studies.41 Because of the significant risk of intracerebral bleeding, cost of therapy, and lack of prospective randomized studies, the impact of these adjunct therapies on morbidity and mortality are still being debated and investigated.39
Vasodilators to improve peripheral and end-organ perfusion have been studied in small populations, and results are variable. Prostacyclin has been reported to improve distal perfusion, whereas nitroprusside infusions and topical nitroglycerin also have been attempted, with some anecdotal success.42
Prevention and Vaccines
Prophylaxis of meningococcal disease is detailed in the American Academy of Pediatrics‘ Red Book and includes the use of rifampin or ceftriaxone for high-risk contacts. These include household contacts especially less than 2 years old, preschool exposures, and those with direct exposure to body fluids. Routine prophylaxis to health care professionals is not recommended.5,31 Quadrivalent polysaccharide vaccine to serogroups A, C, Y, and W-135 have been available since the early 1980s but are largely ineffective. More recently, protein conjugate vaccines have been developed and have shown a strong primary response and has lead to a reduction in nasal carriage.11 Following the introduction of protein conjugate serogroup C vaccine in the United Kingdom, the attack rate decreased by 94% in the immunized population.43 A quadrivalent conjugate vaccine is now available in North America and further interest in the development of a vaccine to serogroup B, that causes approximately 50% of invasive disease worldwide, is ongoing.44
Prognosis
Morbidity and mortality with meningococcus has been difficult to estimate because of multiple serotypes, regional differences, and seasonal variations.45 Fatalities have been reported as high as 75% in epidemic regions in Africa.10 The case fatality ratio in the United States is 10% to 14%, with 11% to 19% morbidity in survivors including complications such as need for skin grafting, loss of limbs/digits, and neurologic disability.44 Several prognostic scores have been developed using multiple clinical and laboratory values. A widely used and validated system is the Glasgow Meningococcal Septicemia Prognostic Score developed by Sinclair and validated by Thomson et al.15,46 Data suggest that early recognition, early antibiotic administration, aggressive resuscitation, and transfer to a pediatric intensive care unit have reduced mortality of severely ill children from the previously reported rate of 35% down to 2% to 13%.14,47,48
Staphylococcus Toxic Shock Syndrome
Etiology and Epidemiology
Staphylococcus aureus is a gram-positive coccus grouped typically in clusters. It is responsible for a wide spectrum of clinical manifestations, including sepsis, pneumonia, cellulitis, arthritis, meningitis, and endocarditis. Staphylococcal toxic shock syndrome (TSS) came into prominence in the early 1980s with the use of super-absorbent tampons and was named menstrual-associated toxic shock.49,50 Thus this form of TSS had a particular predilection for adolescent and young women.51 Now, approximately, half of cases in children are hospital acquired.52,53 Most nonmenstrual cases have been associated with localized musculoskeletal infections, surgery, or insect bites.54–56
Staphylococci produce various toxins. These exotoxins are superantigens causing the activation of large number of T cells. In particular, TSS is linked to an enterotoxin named toxic shock syndrome toxin-1 (TSST-1). TSST-1 accounts for approximately 90% of cases of menstrual toxic shock. Other enterotoxins are responsible for up to 50% of nonmenstrual cases.49,57 TSST-1 acts as a stimulating factor for the release of inflammatory mediators such as interleukin-1β, tumor necrosis factor-α, interleukin-2, and γ-interferon from macrophages and lymphocytes. This massive inflammatory mediator release causes generalized lymphocytic perivasculitis and interstitial edema. This capillary leak syndrome is characteristic of TSS and results in multiorgan dysfunction, including heart, lung, kidney, and brain involvement.58
Clinical Presentation
The Centers for Disease Control and Prevention (CDC) has defined TSS as fever, rash, hypotension, multiorgan involvement, and desquamation with specific criteria (Box 96-1). Onset usually is abrupt, with fever, chills, malaise, and diffuse macular erythroderma. Fever often is remarkably high and resistant, occurring with intense myalgias, vomiting, and diarrhea. The rash is described as a generalized erythematous, deep-red “sunburn” and can be accompanied by conjunctival erythema.59–61 Within 24 hours, cardiovascular depression becomes prominent, with hypotension and decreasing systemic perfusion leading to severe shock and multiorgan dysfunction. Most cases do not cause infective endocarditis unless associated with congenital heart disease. Progressive liver and renal failure are common. The patient may manifest symptoms of diffuse toxic encephalopathy, usually without meningeal signs. Renal failure can be oliguric or nonoliguric. Scaling and desquamation, that are included in the diagnostic criteria, occur with resolution of fever and usually are prominent on the palms and soles.62–63
Box 96–1 Staphylococcus TSS Case Definition
Modified from Centers for Disease Control and Prevention. Toxic-shock syndrome—United States, MMWR 32:201, 1982.
Diagnosis
Differential diagnosis for syndromes that include fever, rash, and multiorgan involvement are numerous and are summarized in Box 96-2. Diagnosis is mainly clinical, but can be supported with inflammatory markers associated with sepsis. The degree of leukocytosis often is not impressive but the percentage of immature cells usually is markedly high.50 Metabolic derangements can support the diagnosis; for example, hypocalcemia and hypophosphatemia are not unique to TSS but can be profound.64 Liver function abnormalities are common. Elevated urea and creatinine concentrations result from prerenal causes initially but also may involve acute tubular necrosis. Persistent lactic acidosis even with restoration of hemodynamics may reflect decreased tissue perfusion and end-organ ischemia.61
Box 96–2 Differential Diagnosis of Staphylococcus TSS
Modified from Chesney PJ: Pediatric infectious disease-associated syndromes. In Fuhrman BP, Zimmerman JJ, editors: Pediatric critical care, St Louis, 1992, Mosby.
Negative blood cultures do not rule out staphylococcus; accordingly, clinical suspicion often must be relied on. Lumbar puncture is not warranted because CSF usually is benign in TSS. Swabs of the primary infection site, such as the postsurgical or vaginal sites can be helpful. TSST-1 antibody assays have been developed but are not recommended, especially because many of the nonmenstrual cases may have other causative endotoxins.63,65
Management
Because the course can be variable and unpredictable, close monitoring and observation are essential when TSS is suspected. Rapid and aggressive fluid administration is required because the severity of end-organ involvement is related to the degree of hypotension and duration of decreased perfusion. Volume requirements may be exceptionally high because of the massive capillary leak characteristic of TSS.66 As a result, acute respiratory distress syndrome (ARDS), myocardial failure, and generalized edema are common. Fluid administration should be guided by close monitoring of blood pressure, central venous pressure, perfusion, and urine output. Other management issues include correction of acid-base abnormalities, electrolyte disorders including hypocalcemia, coagulopathies, renal failure, and fluid overload.67 Mechanical ventilation, renal replacement therapy, and administration of blood products are often necessary.
Two premises to antibiotic therapy for TSS exist. The first is to kill the organism with a bacteriocidal antistaphylococcal β-lactamase–resistant agent such as cloxacillin or vancomycin. The second is to stop enzyme, toxin, and cytokine production with a protein synthesis inhibitor such as clindamycin. Of note, vancomycin is less effective against staphylococcus and should be limited to resistant organisms or allergic patients.49,51,67
Intravenous gammaglobulin (IVIG) has been shown to prevent T-cell stimulation by enterotoxin and may have a role in modulating the inflammatory response.68 No systematically studied trials in humans have described benefits of IVIG, but numerous animal models exist. There are anecdotal examples of its effectiveness, especially in refractory cases.58,69–71 Doses of 150 to 600 mg/kg/day for 5 days or a single dose of 1 to 2 g/kg have been used. In theory, the IVIG must be given early in the course of illness because its role is to prevent rather than treat the systemic inflammatory response.51
Corticosteroids have been used after retrospective studies reported beneficial effects on severity and duration of illness.67,72 However, no further prospective studies have shown specific benefits against the TSST-1 enterotoxin. Therefore steroids usually are reserved for refractory cases where hypotension does not respond to fluids and inotropes. The development of specific antibodies to superantigens is a rapidly developing field.
Prognosis
With aggressive therapy, prognosis is good, with a mortality rate of less than 5%.53,73 Death usually is related to ARDS or refractory arrhythmias. Mortality is higher in nonmenstrual cases.74 Other specific risk factors for higher mortality have not been identified, although increasing age has been linked to poorer outcome.75 Recurrent cases have been noted for both menstrual and nonmenstrual causation.58,61
Invasive Group A β-Hemolytic Streptococcus
Etiology and Epidemiology
Invasive group A β-hemolytic streptococcus (GABHS) is a gram-positive coccus often grouped as chains. GABHS accounts for 3.3% of bacteremia in children.76 Infections resulting from GABHS have become more common, presenting with the two overlapping syndromes of TSS and necrotizing fasciitis.77–79 The CDC has defined TSS caused by GABHS as having prominent features of shock and multiorgan failure (Box 96-3). TSS caused by GABHS is similar to the clinical entity described for Staphylococcus TSS. However, GABHS TSS is more commonly associated with bacteremia and underlying soft-tissue infection compared with staphylococcal TSS.80–82 Necrotizing fasciitis is characterized by extensive local necrosis of soft tissue and skin. GABHS TSS is associated with necrotizing fasciitis in 50% of cases.80,83 Apart from these two entities, invasive GABHS can present with more localized infections such as meningitis, pneumonia, osteomyelitis, septic arthritis, and surgical wound infections. It is important to note that severe invasive GABHS rarely occurs following isolated pharyngitis or scarlet fever; rather, most infections reflect the skin or soft tissue as the portal of entry.84
Box 96–3 Case Definition for Streptococcal TSS
Modified from The Working Group on Severe Streptococcal Infections: Defining the group A streptococcal toxic shock syndrome. Rationale and consensus definition, JAMA 209:390-391, 1993.
The CDC estimates the incidence is 4 to 5 cases per 100,000, with approximately 10,000 cases occurring yearly.81,85 Overall mortality in the pediatric population is approximately 5% to 10% but is much higher in adults.86,87 Approximately 10% of cases are hospital-acquired infections. Infections in clusters or outbreaks, even with close contacts, are uncommon.77 Streptococcal necrotizing fasciitis can occur in otherwise healthy people at any age, but the incidence is higher in young children and the elderly. Risk factors include age younger than 2 years, diabetes mellitus, chronic cardiac or pulmonary disease, immunodeficiency syndromes, intravenous drug use, and malignancy.88 In children, varicella seems to be an increasing risk factor in recent years. GABHS should be suspected as a secondary infection in children with recurrence of fever especially after day 3 of varicella illness or when lesions are more painful.89 Several reports have implicated nonsteroidal antiinflammatory drugs as a risk factor for GABHS infection; however, a causal relationship has not been established.78,90
The pathogenic mechanism is unknown but is thought to be associated with a streptococcal pyrogenic exotoxin, which may act as a superantigen. This stimulates intense activation and proliferation of T lymphocytes and macrophages, resulting in a massive production of cytokines that, in turn, mediate tissue injury, and shock.91
Clinical Presentation
With TSS, most cases present initially with nonspecific flulike symptoms that include fever, chills, and myalgias. Half of the patients develop hypotension within the first 4 hours.92 Multiorgan failure including ARDS and acute renal failure occurs in up to 55% of patients. Mechanical ventilation is necessary in 90% of patients who develop TSS.93 Otherwise, presentation includes myositis, perihepatitis, peritonitis, myocarditis, DIC, and generalized septic shock.86,94
With fasciitis, severe localized pain is the most common initial symptom, usually without correlating physical signs.92 It usually is an acute process with rapid progression. The legs are the most common site of infection, but the abdominal wall, groin, and perianal areas also are frequently affected. In newborns, the site most commonly affected is the umbilicus.85
Approximately 80% of infants eventually develop obvious signs of soft tissue infection, and of these 70% progress to deeper infections requiring surgical debridement. Over the first 24 to 48 hours, the area becomes erythematous and swollen without sharp margins. Skin vesicles and bullae may be bluish and may appear by days 4 to 5, heralding fasciitis. By this time, the area often becomes anesthetic and gangrenous secondary to thrombosis of small blood vessels. Marked swelling and edema may lead to compartment syndrome requiring urgent fasciotomies.85,95
Diagnosis
Signs and symptoms may not be specific for group A streptococcus; thus empiric treatment is initiated before diagnosis is confirmed. Invasive GABHS should be suspected especially when a risk factor such as varicella coinfection or diabetes mellitus is present. Swabbed gram stain and cultures taken from focal lesions probably are the most useful diagnostic tool. Bacteremia is present in 60%, but pending blood culture results should not delay treatment. Often only mild leukocytosis is present, but a left shift is striking.96 Rising serum creatinine kinase concentration has correlated well with deeper soft tissue infections. When necrotizing fasciitis is suspected, magnetic resonance imaging can be helpful in confirming the diagnosis by identifying muscle and underlying fascia inflammation and necrosis.97,98 GABHS was identified in 71% of necrotizing fasciitis cases.99 However, one study in children found GABHS as a single organism in only 25% of the cases; the rest were polymicrobial.61 Other organisms implicated in necrotizing fasciitis are listed in Table 96-1. Approximately half of necrotizing fasciitis cases involve mixed aerobic and anaerobic flora.100
Table 96–1 List of Common Organisms Causing Necrotizing Fasciitis
| Gram-Positive Organisms (Percent) | Gram-Negative Organisms (Percent) |
|---|---|
| Group A streptococci (18–46) | Escherichia coli (8–28) |
| Enterococci (16–34) | Enterobacter (2–12) |
| Coagulase-negative Staphylococcus (15–37) | Pseudomonas (9–20) |
| Staphylococcus aureus (9–37) | Proteus (6–12) |
| Staphylococcus epidermidis (18) | Serratia (2–6) |
| Clostridia (5–21) | Bacteroides (18–48) |
| Mixed gram positives (10) | Mixed gram negatives (16) |
Modified from Cunningham JD, Silver L, Rudikoff D: Necrotizing fasciitis: a plea for early diagnosis and treatment, Mt Sinai J Med 68:258, 2001.
Management
When invasive GABHS (TSS or necrotizing fasciitis) is suspected, aggressive hemodynamic support and prompt antibiotic therapy are essential. A high index of suspicion should encourage initiation of antibiotics specific to GABHS. Frequently, massive amounts of intravenous fluids are required because of the extensive capillary leak.92 Inotropic agents such as dopamine are needed early in order to support adequate cardiac output. Vasoconstrictors such as epinephrine, norepinephrine, and vasopressin should be used with caution because impaired perfusion to necrotic areas may lead to loss of limbs. Milrinone may be a useful adjunct when vasodilation is necessary.95
With TSS, both GABHS and S. aureus should be covered with broad-spectrum antibiotics. With a microbiologic diagnosis, the antibiotics can be tailored later. Group A streptococci in general are extremely sensitive to β-lactam antibiotics, but invasive GABHS infections seem to respond less well to the penicillins alone.101,102 With GABHS identified, penicillin G 200,000 to 400,000 U/kg/day in four to six divided doses can be administered. Clindamycin as an adjunct has proved effective at doses of 25 to 40 mg/kg/day in three to four divided doses.95,103 Clindamycin works by inhibiting protein synthesis, thus suppressing bacterial toxin production and decreasing penicillin-binding protein synthesis. Clindamycin also has been shown to modulate the immune response.101 Though no controlled clinical trials have demonstrated improved outcome with the addition of clindamycin, it is recommended in combination with a third generation cephalosporin as initial therapy.90
With necrotizing fasciitis, prompt surgical exploration and aggressive debridement are critical, with involvement of general, orthopedic, and plastic surgeons. By the time clinical signs are present, saving viable tissue may not be possible, but debridement is necessary to prevent the progression of inflammation and necrosis.98 The patient often is returned to the operating room daily for inspection until the infection is adequately controlled. Fasciotomies often are needed in the presence of compartment syndrome in order to facilitate adequate perfusion.102 Amputation may be necessary and should be undertaken early if necessary. Then, daily dressing changes can continue with close monitoring of surgical margins. Often daily magnetic resonance imaging is warranted.98,100
Antibodies specific for the toxin do not exist, but use of IVIG 1 to 2 g/kg in a single dose has been effective in case reports in both TSS and necrotizing fasciitis.69–71,104,105 The possible mechanism may involve prevention of T-cell proliferation and reduction of cytokine release. Some anecdotal reports of the benefits of hyperbaric oxygen also exist but are inconclusive.106 No controlled trials have demonstrated the efficacy of either of these therapies.
Prognosis
Though incidence of fasciitis may be declining with the use of varicella vaccination, mortality rates for TSS still vary from 30% to 70%.85,86,107,108 For necrotizing fasciitis, mortality rates range from 6% to 36%.100 Morbidity is high, including surgical intervention in greater than 50% of suspected fasciitis cases.84 Irreversible renal impairment is seen in up to 10%. The CDC estimated 1350 deaths related to GABHS in 2007.81 Unfavorable outcome has been shown to be affected by delayed diagnosis and by the presence of organ failure at the time of admission.109

Full access? Get Clinical Tree