135 Infections of Skin, Muscle, and Soft Tissue
 Necrotizing Soft-Tissue Infections
Necrotizing Soft-Tissue Infections
NSTIs represent a spectrum of infectious processes that are extensive and rapidly progressive. Based on the depth of skin and soft-tissue involvement, NSTIs are divided into three categories: necrotizing cellulitis, necrotizing fasciitis, and myonecrosis. Table 135-1 shows the classification of NSTIs. The sine qua non of these infections is necrosis of subcutaneous tissue, fascia, and muscle, with widespread undermining of the skin. The lack of anatomic boundaries and the fact that the infection is deep to the skin helps account for the severity of the infection as well as the frequent delay in its recognition. The trunk, extremities, and perineum are the most common sites of NSTIs, but other anatomic sites may be involved. For example, intraabdominal abscess, bowel perforation, and pancreatitis can present as necrotizing infection of the abdominal wall or extend into the thigh along the psoas muscle.1,2 Similarly, cervical fasciitis due to dental or neck abscess can extend to the mediastinum.
TABLE 135-1 Classification of Necrotizing Skin, Soft-Tissue, and Muscle Infections
| Disease | Bacteriology | Comments |
|---|---|---|
| Necrotizing Cellulitis | ||
| Clostridial cellulitis | Clostridium perfringens | Local trauma, recent surgery; fascial/deep muscle spared |
| Nonclostridial cellulitis | Mixed: Escherichia coli, Enterobacter, Peptostreptococcus spp., Bacteroides fragilis | Diabetes mellitus predisposes; produces foul odor |
| Meleney’s synergistic gangrene | Staphylococcus aureus, microaerophilic streptococci | Rare infection; postoperative; slowly expanding, indolent, ulceration in superficial fascia |
| Synergistic necrotizing cellulitis | Mixed aerobic and anaerobic, including B. fragilis, Peptostreptococcus spp. | Diabetes mellitus predisposes; variant of necrotizing fasciitis type I; involves skin, muscle, fat, and fascia |
| Necrotizing Fasciitis | ||
| Type I | Mixed aerobic and anaerobic; staphylococci, B. fragilis, E. coli, group A streptococci, Peptostreptococcus spp., Prevotella, Porphyromonas spp., Clostridium spp. | Usually requires a breach in the mucous membrane layer either through surgery or penetrating injuries or from chronic medical conditions such as diabetes, peripheral vascular disease, malignancy, and anal fissures |
| Type II | Group A streptococci | Increasing in frequency and severity since 1985; very high mortality; often begins at site of nonpenetrating minor trauma such as a bruise or muscle strain but often no identified precursor |
| Predisposing factors: blunt/penetrating trauma, varicella (chickenpox), intravenous drug abuse, surgical procedures, childbirth, NSAID use | ||
| Myonecrosis | ||
| Clostridial myonecrosis | Clostridium spp. | Predisposing factors: deep/penetrating injury, bowel and biliary tract surgery, improperly performed abortion and retained placenta, prolonged rupture of the membranes, and intrauterine fetal demise or missed abortion in postpartum patients. Recurrent gas gangrene occurs at sites of previous gas gangrene. |
| Streptococcal myonecrosis | Streptococci | |
| Special Type of Necrotizing Soft-Tissue Infection | ||
| Fournier’s gangrene | Polymicrobial, with E. coli the predominant aerobe and Bacteroides the predominant anaerobe. Other microflora: Proteus, Staphylococcus, Enterococcus, aerobic and anaerobic Streptococcus, Pseudomonas, Klebsiella, and Clostridium | Necrosis of the scrotum or perineum that starts with scrotal pain and erythema and rapidly spreads onto anterior abdominal wall and gluteal muscle. It is more often seen in diabetics and can be associated with trauma. |
Pathogenesis
Host Resistance
As shown in Table 135-2, individuals who are immunocompromised or have chronic diseases are more likely to develop necrotizing skin and soft-tissue infections than those without such medical problems.
TABLE 135-2 Factors Predisposing to Necrotizing Soft-Tissue Infections
Bacterial Pathogens
There are specific bacteria that are more likely than others to cause NSTIs, as shown in Table 135-1. Although necrotizing cellulitis and fasciitis may be caused by a single bacterial pathogen such as group A Streptococcus, Vibrio spp., or zygomycetes, about 80% of necrotizing cellulitis or fasciitis results from polymicrobial infections with synergistic facultative aerobes and anaerobic gas-forming organisms. An average of 4.4 organisms are isolated from polymicrobial necrotizing infections.3 The former includes gram-positive and gram-negative aerobes such as Streptococcus pyogenes, Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, or Pseudomonas aeruginosa, and the latter includes Clostridium perfringens, Bacteroides fragilis, and Peptostreptococcus.4 Certain predisposing conditions can be correlated with specific bacteria—for example, trauma with Clostridium spp., diabetes mellitus with Bacteroides spp., S. aureus, and Enterobacteriaceae, and immunosuppression with Pseudomonas spp. and Enterobacteriaceae.5
Clinical Manifestations and Diagnosis
The critical aspect of diagnosing NSTIs is maintaining a high index of suspicion, which allows for early recognition of the nonlocalized necrotizing nature of the infection and the need for surgical intervention. Although necrotizing cellulitis and fasciitis may occur after significant tissue trauma or a relatively trivial injury, up to 40% of NSTIs have no identifiable cause. NSTIs with identifiable barrier failure are more likely to be polymicrobial and are easier to diagnose than the more virulent infections caused by a single organism. In necrotizing cellulitis, gas is invariably found in the skin, but the fascia and deep muscle are spared. Early clinical findings are similar to those of common wound infections, including local edema (89%), erythema (30%), fever (71%), and local cutaneous anesthesia (27%) due to cutaneous nerve necrosis.6 These are followed by gangrenous skin changes with rapid extension beyond the borders of the original infection. Synergistic polymicrobial necrotizing fasciitis is characterized by “dishwater pus.” Patients usually have high fever, but no obvious source of clinical infection can be detected. Pain in the area of infection is usually out of proportion to the physical findings. As the infection progresses, patients develop shock and multiple organ failure. Mortality rates are high, with necrotizing fasciitis being fatal in 23.5% of cases.7
Management
Antibiotics
Although there are no data from clinical trials establishing the benefit of combined therapy in type II necrotizing fasciitis (group A streptococci), penicillin G combined with clindamycin is the antibiotic therapy of choice. Clindamycin, but not metronidazole, is recommended not for its antianaerobic properties but because of its additional activity against gram-positive organisms, including specific inhibition of toxin production.8 Cefotaxime and ceftriaxone are acceptable alternatives. For patients allergic to penicillin, vancomycin is the recommended treatment.
Surgical Intervention
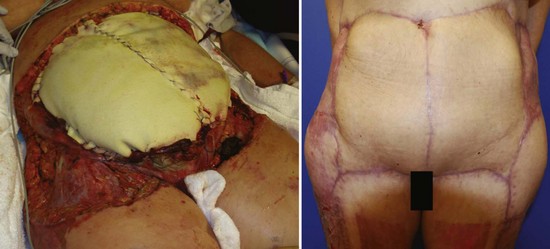
Early surgical débridement is critical in the management of NSTIs. Aggressive surgical excision of all involved tissue with a margin of normal-appearing tissue is mandatory. All necrotic tissue should be excised back to healthy bleeding margins. Additional incisions parallel to cutaneous nerves and blood vessels may be used to assess fascial viability without elevating the skin. The wound should be frequently reexamined for viability of tissue and repeat operative débridement is frequently required. Aggressive fascial débridement of abdominal surgical wounds may necessitate the use of prosthetic material to replace an abdominal wall defect, as depicted in Figure 135-1. In Fournier’s gangrene and perineal/perirectal NSTI, a colostomy for fecal diversion may be necessary to keep the wound clean. The testes generally survive because their blood supply is usually spared, but they may need to be temporarily implanted in the soft tissue of the medial thighs if the scrotum must be débrided. On rare occasion, NSTI of the extremities may require amputation.
Adjunctive Therapy
Hyperbaric Oxygen
The use of hyperbaric oxygen (HBO) in NSTIs is controversial. Although there are no randomized prospective studies of HBO in these infections, in vitro data and reviews of clinical series seem to show beneficial effects of HBO when combined with antibiotics and surgical débridement in the management of clostridial infection.9,10 Hyperbaric oxygen is toxic to clostridia and inhibits bacterial growth, blocks production of alpha toxin, and preserves marginally perfused tissue. Debate also exists about the use of HBO for nonclostridial necrotizing skin and soft-tissue infection. In one report, the addition of HBO to the surgical and antimicrobial treatment of nonclostridial necrotizing fasciitis significantly reduced mortality and the need for débridement.9
Intravenous Immunoglobulin
Intravenous immunoglobulin (IVIG) has been administered to patients with streptococcal and staphylococcal toxic shock syndrome and may be efficacious in the treatment of this toxin-mediated disorder. Some studies have demonstrated IVIG has some beneficial effect in the treatment of NSTIs, theoretically owing to its neutralization of circulating clostridial toxins and streptococcal superantigens.11 However, a large multicenter retrospective cohort study of children with streptococcal toxic shock syndrome showed no improvement in outcomes with administration of IVIG.12 There is no clear consensus at this time regarding the efficacy of IVIG.
 Important Soft-Tissue Infections of the Head and Neck
Important Soft-Tissue Infections of the Head and Neck
Ludwig’s Angina
In 1836, German physician Wilhelm Frederick von Ludwig described five patients with gangrenous induration of the connective tissues of the neck that progressed rapidly to involve the tissues covering the muscles between the larynx and the floor of the mouth.13 Ludwig’s angina is a potentially life-threatening, rapidly progressive, diffuse “woody” or brawny cellulitis of the submandibular and sublingual spaces that occurs most often in young adults with dental infections.
Pathogenesis
In adults, 50% to 80% of cases of Ludwig’s angina are caused by dental caries, and the disease has a mortality rate of 5% to 10%.14 Submandibular and sublingual spaces freely communicate, and with involvement of the deep cervical fascia, infection may spread rapidly, with grave consequences. Extension along the carotid sheath or the retropharyngeal space can cause mediastinitis.15 Infection is commonly caused by oral cavity anaerobes such as Fusobacterium, anaerobic streptococci, Bacteroides, spirochetes, and hemolytic Streptococcus organisms, although the infection may be mixed with Staphylococcus and Streptococcus, Klebsiella, or a combination of aerobic or anaerobic organisms.16 The presence of anaerobes commonly accounts for the occurrence of gas in the tissues.
Clinical Manifestations
The diagnosis of Ludwig’s angina is usually made clinically according to three criteria: (1) presence of cellulitis with little or no pus in both submandibular and sublingual spaces; (2) presence of gangrene with serosanguineous putrid fluid; and (3) rapidly spreading cellulitis in connective tissue, fascia, and muscles, without glandular tissue and lymphatic involvement.17
Management
Control of Airway
Progression from the first findings of symptoms to asphyxia may occur rapidly over several minutes to a few hours. Therefore, airway protection is a critical component of initial management. Stridor, tachypnea, dyspnea, inability to handle secretions, and agitation are all indicative of impending airway loss. In the past, the standard of care for Ludwig’s angina was early emergency intubation or tracheostomy to protect the airway. However, this practice has been gradually abandoned. Recent data show that most cases can be managed initially by close observation in a critical care unit and intravenous antibiotics.18 If an artificial airway is required, flexible fiberoptic-guided nasotracheal intubation is the preferred method of airway control. Tracheostomy, under local anesthesia and performed through the cellulitis, is still the most widely recommended means of obtaining a surgical airway.

Full access? Get Clinical Tree