Fig. 24.1
Chest X-ray
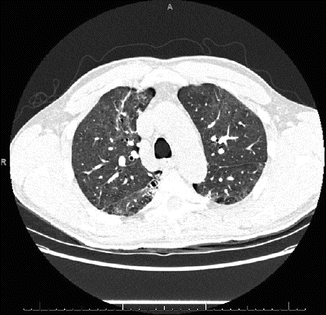
Fig. 24.2
High-resolution CT scan
Question
Should the patient’s antimicrobial regimen be changed? What diagnostic test should be performed?
Answer
The patient’s antimicrobial regimen should be expanded empirically to cover Pneumocystis jirovecii (e.g. trimethoprim-sulfamethoxazole) given (1) his risk factors (cyclosporine and corticosteroids), (2) his consistent CT scan (interstitial infiltrate with cystic changes), (3) his hypoxemia disproportionate to radiographic infiltrate, (4) his lack of clinical response to an empiric regimen adequate for community-acquired pneumonia, and (5) the fact that empiric therapy does not compromise the diagnostic yield of subsequent bronchoscopy in the diagnosis of Pneumocystis pneumonia [1]. A lower respiratory tract specimen should be acquired, via bronchoscopy or mini- bronchoalveolar lavage (BAL); lavage fluid should be tested for gram stain and culture, respiratory virus polymerase chain reaction (PCR), fungal culture, galactomannan, acid-fast stain and culture, and Pneumocystis PCR.
The patient underwent flexible bronchoscopy, and a positive Pneumocystis PCR assay confirmed the diagnosis. The patient received intravenous trimethoprim-sulfamethoxazole, and oxygenation gradually improved over the next 5 days. The patient was ultimately extubated and recovered full lung function. After 21 days of treatment, the patient’s trimethoprim-sulfamethoxazole was changed to the prophylactic dose (1 double-strength tablet [160/800] once daily) for the duration of his immunosuppression.
Principles of Management
Presentation
Pneumonia is a common and morbid complication of immunosuppression, whether due to primary immunodeficiency or, more commonly, secondary to a systemic disease process or its treatment. The presentation of pneumonia among immunosuppressed patients is often more subtle, indolent and atypical than among immunocompetent patients [2]; the same immune deficits that permit microbial reproduction in the lower respiratory tract can decrease the intensity of fever, sputum production, or radiographic infiltrates. Immunosuppressed patients are often vulnerable to competing or concurrent non-infectious lung processes such as cardiogenic edema (e.g. among patients receiving cardiotoxic chemotherapy or aggressive hydration with chemotherapeutic regimens), medication toxicity (e.g. among patients receiving bleomycin or methotrexate), radiation pneumonitis, or malignancy (e.g. Kaposi’s Sarcoma among patients with Human Immunodeficiency Virus [HIV]/Acquired Immunodeficiency Syndrome [AIDS]).
Etiology
The presence and persistence of microbes in the respiratory tract are determined by the balance of microbial immigration, elimination and local microbial growth conditions [3, 4], all of which are altered in immunosuppressed patients. The microbiota of the upper respiratory tract (the primary source community for migration of microbes to the lungs [4, 5]) are altered by systemic immunosuppression, whether by underlying disease (e.g. HIV/AIDS) [6] or immune-suppressing medications [7]. Impairment of innate and adaptive immunity decreases the elimination rate of transient microbes, increasing the likelihood of persistent reproduction, and makes the microbial growth conditions of the lung environment more hospitable to dysregulated reproduction [3]. Each patient’s specific constellation of immune deficits predisposes him/her to a select number of opportunistic pathogens (Table 24.1). Consideration of each patient’s candidate pathogen profile is critical to the appropriate selection of empiric antimicrobial therapy. Despite the wide breadth of potential pathogens in this population, the most common culprits remain the bacteria and viruses responsible for community-acquired pneumonia (e.g. Streptococcus pneumoniae) [9], which should be covered by any empiric regimen. Coverage for atypical organisms (Mycoplasma spp., L. pneumophila and C. pneumoniae) is warranted in community-dwelling patients until a specific pathogen is identified.
Table 24.1
Correspondence of immunodeficiency and susceptibility to respiratory pathogens
Immune defect | Disease examples | Iatrogenic examples | Organisms to suspect | |
|---|---|---|---|---|
Innate immunity | Neutrophil abundance | Leukemia Parvovirus infection Agranulocytosis | Chemotherapy Methotrexate Clozapine | Gram-negative bacilli Staphylococcus spp. Fungi (e.g. Aspergillus spp.) |
Neutrophil function | Chronic granulomatous disease Cirrhosis Uremia | Anti-TNF agents [8] | Staphylococcus aureus Fungi (e.g. Aspergillus spp.) | |
Adaptive immunity | T-cell abundance and function | HIV/AIDS Lymphoma Primary immunodeficiency | Chemotherapy Corticosteroids Calcineurin inhibitors Anti-T-cell antibodies | Pneumocystis jirovecci Cryptococcus spp. Intracellular bacteria (e.g. Legionella spp.) M. tuberculosis Viruses (CMV, HSV, VZV) |
B-cell abundance and function | Multiple myeloma Primary immunodeficiency | Rituxumab | Encapsulated bacteria: S. pneumoniae, H. influenzae | |
Diagnosis
Chest x-rays are of notoriously poor sensitivity in identifying pneumonia among immunocompromised patients; in one large series, the majority of neutropenic patients with infiltrates on thin-sliced CT scans had no detectable abnormality on chest radiograph [10]. High-resolution CT scan is often helpful for confirming the presence of infection, guiding site selection for bronchoalveolar lavage, and directing empiric therapy based on imaging characteristics. The presence of cavitation is associated with Mycobacterium spp., Nocardia spp., Aspergillus spp. and P. jirovecci.; interstitial infiltrates suggest viral (e.g. Cytomegalovirus [CMV]) pneumonia or Pneumocystis; dense consolidation implies either bacterial pathogens or Aspergillus spp.. Serologic tests are of decreased utility in immunocompromised patients, especially in patients with impaired T-cell and B-cell immunity (Table 24.1), whereas antigen-based testing (e.g. Streptococcus and Legionella urinary antigens, Cryptococcus serum antigen testing) can be useful. An aggressive approach to sampling the lower respiratory tract (via bronchoscopy or miniature bronchoalveolar lavage [“mini-BAL”]) is warranted, as the spectrum of potential pathogens usually exceeds any reasonable empiric antimicrobial regimen. Depending on the patient’s degree and type of immunosuppression, lower respiratory tract specimens should be tested for gram stain and bacterial culture, fungal culture, acid fast stain and culture, respiratory viral PCR, CMV antigen, galactomannan, Pneumocystis PCR. Recommended diagnostic tests by specimen site are listed in Table 24.2.
Table 24.2
Diagnostic testing in immunocompromised patients with suspected pneumonia
Specimen | Diagnostic tests |
|---|---|
Bronchoalveolar lavage fluid | Cell count and differential |
Gram stain and bacterial culture | |
Fungal stain and culture | |
Acid-fast bacteria stain and culture | |
Respiratory virus PCR | |
Pneumocystis jirovecci PCR | |
CMV antigen | |
Galactomannan | |
Serum | Bacterial culture

Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




