TOPICS
1. Signs, Symptoms, and Diagnosis of HCM
4. Dynamic obstruction of the LVOT
5. Anesthetic management of the HCM patient for noncardiac surgery
6. Anesthetic management for surgical repair of HCM
7. The Surgical approach to HCM repair
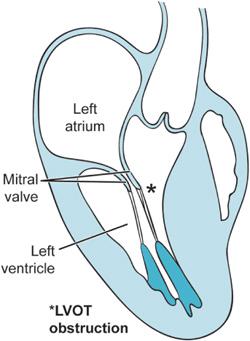
Previous chapters have discussed how fixed obstructions to blood flow through the heart can lead to significant morbidity and mortality. Aortic and mitral stenosis are two examples of lesions, which may prevent the heart from effectively pumping blood. Hypertrophic cardiomyopathy (HCM) likewise can prevent the heart from pumping blood but in a dynamic rather than fixed way. With each beat the hypertrophied septum of the HCM patient together with the anterior mitral valve leaflet prevent the heart from ejecting blood as they obstruct the left ventricular outflow tract (LVOT) (Figure 10–1). Hence, this condition in the past was known as hypertrophic obstructive cardiomyopathy (HOCM). Failure to eject enough blood from the heart leads to syncope, dyspnea, and, at times, sudden death.
Although rare, cardiac tumors and other masses can interfere with valve function, produce emboli, and dynamically obstruct blood flow through the heart and prevent ejection into the systemic circulation. This chapter will examine these different conditions, which are nonetheless linked by their dynamic ability to prevent the heart from properly functioning.
Figure 10–1. The midesophageal long-axis view is presented here in outline form. As a consequence of the hypertrophied interventricular septum, flow patterns within the heart are altered such that the anterior leaflet of the mitral valve is drawn during ventricular systole into the LVOT producing obstruction. This is known as systolic anterior motion of the mitral valve (SAM).
SIGNS, SYMPTOMS, AND DIAGNOSIS OF HCM
HCM is an autosomal dominant trait; roughly one-half of the patients have a blood relative afflicted with HCM.1 The disease can affect both males and females. It is estimated that HCM presents in 1:500 of the general adult population and that these patients are at increased risk for sudden cardiac death (SCD).2 Certainly, many patients with HCM are not detected and can present with SCD as the first manifestation of their cardiac disease.
Symptoms of HCM include: dyspnea, exercise intolerance, palpitations, syncope, chest pain, and SCD. HCM can manifest in both the left and the right heart; however, it is overwhelmingly a disease of the left ventricle. HCM can occur with and without LVOT obstruction.3 In non-obstructive HCM disease the patients develop a hypertrophied myocardium with diastolic dysfunction. As previously discussed in Chapter 2, diastolic dysfunction occurs when the heart is unable to normally relax during diastolic filling. Subsequently, impaired ventricular compliance leads to an increased left ventricular end-diastolic pressure (LVEDP), elevated pulmonary arterial pressures, increased pulmonary congestion, and decreased coronary perfusion pressure (CPP). Such patients can develop angina, dysrhythmias, systolic heart failure, and sudden death in the absence of any LVOT obstruction. Nonetheless, patients in whom HCM disease produces an obstruction of the LVOT are at a significantly increased risk of death and/or severe heart failure.3
Clinically, the diagnosis of HCM with dynamic LVOT obstruction is made by the auscultation of a late systolic murmur. The murmur is heard in late systole as the anterior leaflet of the mitral valve abuts the hypertrophied septum during systole (Videos 10–1 and 10–2). This systolic anterior motion (SAM) of the mitral valve provides the source of dynamic systolic obstruction in these patients (Figure 10–2A and 2B). Various clinical maneuvers can be employed to increase the murmur by reducing the venous return to the heart (eg, Valsalva maneuver). By reducing venous return to the heart, the ventricles fill to a lesser degree. In the HCM patient, the fuller the heart, the less likely it is that during systole the mitral valve will be able to obstruct the LVOT. In the underloaded heart, however, the anterior leaflet of the mitral valve can more readily obstruct the ejection of blood.
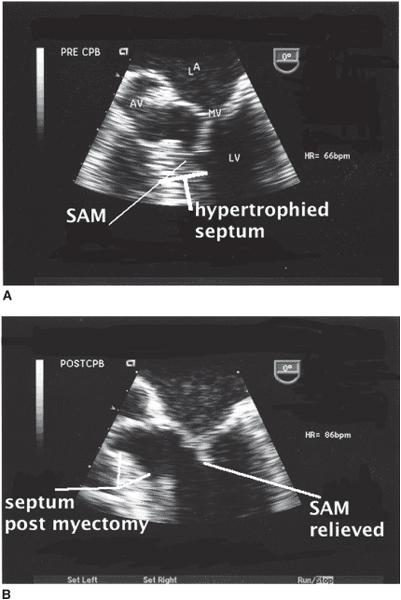
Figure 10–2. A. The midesophageal four-chamber view seen here focuses upon the left ventricular outflow tract. The upper panel demonstrates systolic motion of the anterior leaflet of the mitral valve (SAM). B. The same image is obtained following surgical myectomy. SAM is no longer present and the area of resected myocardium is identified. (From: Balaram S, Sherrid M, De Rose J, et al. Beyond extended myectomy for hypertrophic cardiomyopathy: the resection-plication-release (RPR) repair. Ann Thorac Surg. 2005;80:217-233, with permission.)
Not surprisingly, echocardiography is best suited to make the diagnosis of HCM disease.1 When ventricular wall thickness in diastole is greater than 14 mm the patient is thought to have HCM disease assuming there are no other conditions that would produce ventricular hypertrophy (eg, aortic stenosis or systemic hypertension). Symptomatic patients routinely have thickened interventricular septums of 20 to 30 mm.1
PATHOLOGY OF HCM
HCM is a genetic disease. Mutations in the genes, which code for the cardiac sarcomeres and their supporting proteins, have been implicated as causes of HCM.1,2 Mutations in the beta-myosin heavy chain and myosin-binding protein C represent upward of 50% of the genotyped patients.2 However, other cardiac protein mutations can also be associated with HCM. Additionally, the phenotypic expression of the HCM patient is variable. Thus, even in families with the same mutation, the variations in phenotypic expression can lead to radically different outcomes.1
At the cellular level, the myocardium of the HCM is a mix of oddly shaped myocytes and fibrotic tissue. The myocytes of the HCM patient lack the neat parallel array of normal ventricular muscle tissue.1
This abnormal myocardium can become quite noncompliant leading to diastolic dysfunction. As the LVEDP increases, pressure is transmitted through the left atrium to the pulmonary vasculature resulting in elevated pulmonary arterial pressures (PAP). The HCM patient can develop dyspnea with minimal exertion. Thus, HCM patients can be symptomatic without having LVOT obstruction.
Systolic function is often initially maintained in the HCM patient. However, over time in a small percentage of patients, the heart’s systolic pumping ability fails.3 In HCM patients especially if LVOT obstruction is present, the increased LV work to overcome elevated pressures will lead to increased wall stress, ischemia, cell death, ventricular fibrosis, and heart failure.3
Mitral regurgitation frequently occurs in HCM with obstruction of the LVOT. Some of the causes responsible for the development of mitral regurgitation are elevated intraventricular pressures, systolic anterior motion (SAM) of the mitral leaflets in the presence of LVOT obstruction, abnormally positioned papillary muscles, and redundant mitral leaflets. Mitral regurgitation may result in left atrial dilatation, atrial fibrillation, decreased stroke volume, and further impairment of the ventricular function (Figure 10–3).
SCD is one of the most dreaded complications of HCM.4 Consequently, many patients with this condition are provided with an implantable cardioverter defibrillator (ICD) after a risk stratification and selection process.
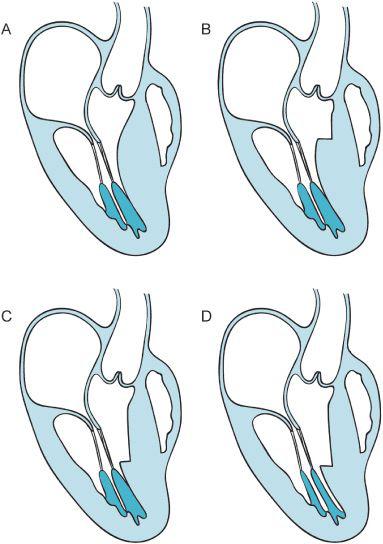
Figure 10–3. Image “A” presents a schematic of the long-axis view of the HCM patient. Flow dynamics associated with the enlarged ventricular septum push the anterior leaflet of the mitral valve into the outflow track. In window “B” the surgeon has performed a small myectomy, however, the flow within the heart can still produce SAM. Conversely in windows “C” and “D” an extensive myectomy is performed and the papillary muscles are partially excised to direct the flow of blood more anteriorly toward the LVOT rather than posteriorly. The coaptation point of the mitral valve leaflets is thus more posterior in the ventricle away from the anteriorly placed LVOT. (Redrawn from: Sherrid M, Chaudury F, Swistel D, et al. Obstructive hypertrophic cardiomyopathy; echocardiography, pathophysiology, and the continuing evolution of surgery for obstruction. Ann Thorac Surg. 2003;75:620-632, with permission.)
MEDICAL THERAPY FOR HCM
The majority of patients with HCM are managed medically when symptoms warrant.1 Asymptomatic patients are routinely managed expectantly; however, ICD placement may be considered in those with a strong family history of SCD. Medical therapy is centered primarily upon determining the nature of HCM encountered. The non-obstructive HCM patient presents with signs and symptoms of diastolic dysfunction.1 Both beta-blockers and calcium antagonists have been used in this setting with variable results. It is possible that these agents improve symptoms by reducing the incidence of myocardial ischemia in the HCM patient. Patients presenting with signs of heart failure in the non-obstructive HCM patient can be treated with judicious administration of diuretics to relieve symptoms of pulmonary congestion. However, reduced ventricular volumes could prove problematic as the noncompliant, dysfunctional ventricle requires an adequate SV to generate an acceptable cardiac output. Rhythm disturbances such as atrial fibrillation should also be corrected in order to permit adequate LV filling during diastole.
Patients with obstructive HCM can develop intraventricular pressure gradients during systole. Gradients across the LVOT of over 100 mm Hg can be detected between different regions of the LV cavity (Video 10–3). Indeed, the presence of obstruction with gradients as low as 30 mm Hg is predictive of worse outcomes and increased mortality in the HCM patient population.3 Therapy is aimed at reducing myocardial contractility in order to reduce the LVOT gradient during systole. Beta-blockers, verapamil, and disopyramide are employed to this end. These agents have negative inotropic effects and reduce the systolic gradient. Likewise, efforts are made to maintain a large, well-filled left ventricle. Any situation leading to a reduction in the size of the left ventricle (eg, hypovolemia, increased myocardial contractility, and decreased systemic vascular resistance) will increase the dynamic obstruction during ventricular systole.
DYNAMIC OBSTRUCTION OF THE LVOT
HCM patients can be symptomatic whether they have the obstructive form or the non-obstructive form of cardiomyopathy. It is important to realize that both presentations can lead to perioperative complications resulting from arrhythmia, hypotension, and even sudden cardiac death. Although the cardiac anesthesiologist is likely to encounter that subset of obstructive HCM patients not amenable to medical therapy, it is critical to understand that the HCM patient may undergo a variety of procedures and have varying anesthetic requirements.
What differentiates the obstructive form of HCM from the non-obstructive form is the dynamic development of a pressure gradient across the LVOT.
The LVOT provides the pathway for oxygenated blood as it leaves the LV cavity and is ejected out of the aortic valve. The systolic anterior motion of the mitral valve in the LVOT is the most common cause of dynamic obstruction generating significant pressure gradients (> 50 mm Hg) across the LVOT. The hypertrophied bulge of the intraventricular septum at the level of the LVOT may direct the flow in the heart in such a way that it drags the anterior leaflet into the LVOT producing obstruction.5 The development of increased LV intracavitary pressure can soon lead to mitral valve incompetence and worsening symptoms of systolic and diastolic heart failure.
ANESTHETIC MANAGEMENT OF THE HCM PATIENT FOR NONCARDIAC SURGERY
Only a minority of HCM patients is brought to surgery for myectomy and/or mitral valve replacement. Many patients will nonetheless require anesthesia at some point for noncardiac surgery. Management is directed at minimizing the degree of outflow obstruction, lessening the impact of diastolic dysfunction, and controlling arrhythmias. Both general and neuraxial anesthesia techniques can produce wide swings in hemodynamics. As mentioned, during anesthesia induction in the patient with a fixed obstruction to systolic ejection, such as aortic stenosis, it often becomes necessary to administer fluids and vasoconstrictors to prevent hemodynamic instability. In the patient with dynamic obstruction, the greater the decrease in venous return the worse the degree of obstruction. Agents, which decrease sympathetic tone and peripheral vascular resistance, likewise can increase the degree of dynamic obstruction, as the heart is free to contract against less resistance. An increase in heart rate associated with laryngoscopy, intubation, and surgical stimulation should be avoided, as this will decrease the time of LV diastolic filling and systolic ejection resulting in worsening of the degree of dynamic obstruction and hypotension. Consequently, these patients are frequently managed with fluid and vasopressor administration at the time of induction and intraoperative arterial and TEE monitoring. Short-acting beta-blockers such as esmolol can be used to reduce myocardial contractility, decrease the heart rate, and counteract the effects of increased catecholamine release during intubation, emergence, and other periods associated with surgical stress.
Neuraxial anesthesia approaches can be employed2 in these patients and have also been used in the patient with HCM for labor analgesia.2 The decrease in sympathetic tone with its associated reduction in venous return and decrease in peripheral vascular resistance makes the use of neuraxial techniques potentially deleterious. Careful titration of local anesthetic to obtain the appropriate level of anesthesia is clearly important if these approaches are to be safely employed. Likewise, invasive monitoring may prove helpful in this setting in order to detect acute deteriorations. Regional techniques which leave sympathetic tone relatively unaffected (eg, peripheral nerve blocks) could be of use where indicated in the HCM patient.
HCM patients frequently have undergone the placement of an ICD. The peri-operative management of these devices is discussed in Chapter 15.
Anesthetic management may be further complicated by diastolic dysfunction. Additionally, patients often have some degree of mitral regurgitation, which further increases LAP, PCOP, and pulmonary congestion. Treatment is aimed at reducing the degree of dynamic obstruction, favoring adequate systolic ejection and thereby, reducing MR and pulmonary congestion. Although diuresis to unload the heart might be desirable in the patient with a high grade of diastolic dysfunction, care must be taken not to reduce ventricular volume as to worsen the obstruction across the LVOT.
ANESTHETIC MANAGEMENT FOR SURGICAL REPAIR OF HCM
For those patients who do not benefit from medical management, there are surgical approaches to relieve dynamic obstruction and restore adequate mitral valve function. Anesthetic management remains centered upon minimizing the degree of LVOT obstruction during the hemodynamic shifts associated with general anesthesia. Monitoring is similar to that employed in any cardiac surgical case requiring CPB. The utility of the PA catheter in this setting is unproven. However, TEE is essential to the performance of the operation. In this procedure, TEE is not merely a monitor of ventricular function or an additional surveillance tool—it is essential for the surgical management of the patient.5,6 There is close interaction between the anesthesiologist and the surgeon during the surgery.
A variety of induction and maintenance agents can be employed in these patients. More important than the drug choice for anesthesia induction is directing therapy toward maintaining an adequately volume-loaded and less-contractile heart. Catecholamine release associated with inadequate anesthetic depth can increase obstruction secondary to tachycardias and augmented myocardial contractility.
As mentioned in the previous section, hemodynamic derangements such as hypotension or tachycardia at the time of induction should be treated promptly using vasoconstrictors, fluids, or short-acting beta-blockers. Antiarrhythmics and beta-blockers should be continued perioperatively. Intraoperative therapeutic interventions may be monitored using TEE guidance to demonstrate relief of obstruction and reduced pressure gradient.
Video 10–3 demonstrates the LVOT being obstructed by the anterior leaflet of the mitral valve abutting the bulging septum. The deep gastric view is employed and as such there is a good alignment between the LVOT and the Doppler beam. Using continuous wave Doppler (CWD), it is possible to determine the velocity of flow across the obstruction. Videos 10–3 displays the time-velocity integral of a patient with a severe dynamic gradient. Using Bernoulli equation, it is possible to calculate the peak gradient present across the LVOT:
Pressure gradient = 4V2

Full access? Get Clinical Tree