The unpredictable and episodic nature of many of the primary headaches introduces practical difficulties into research in this field, in imaging studies as well as in therapeutic intervention studies. The difficulties include getting patients to travel to the research center while suffering with severe pain or nausea and vomiting from migraine. This delays the onset of any therapy in interventional studies and introduces a selection bias in that those most debilitated do not participate in the study because they are unable to travel. It also means that the attack cannot be followed from the very onset. The pathophysiology and clinical manifestations of the headache may change throughout the attack; for example, in migraine there may be a premonitory phase prior to the headache or aura phase. These problems have fueled the search for good human headache models. Although animal models may take us far in understanding certain steps of the pathophysiology of primary headaches, human models are of great importance in studying the complete disease process from initiation of attack to treatment and pain relief.
The question then arises as to what makes a good headache model. One major issue in developing these models is to agree on the diagnoses of the patients studied. The introduction of the diagnostic criteria for headache disorders of the International Classification of Headache Disorders (ICHD-2) (
1) has helped tremendously in the comparability and validation of the different models. Also, the systematic use of these criteria makes it possible to compare the distinct symptomatology of spontaneous headache to induced headaches to allow an objective diagnosis to be made. Unfortunately, not all of the previous headache models have used the ICHD criteria, which may limit comparison.
An effective headache model enables the headache to be followed in a controlled environment throughout the attack. The model needs to be reliable, reproducible, and validated. Safety is obviously an issue, and it is preferable to use a well-known compound that is easy to administer, has few reversible and tolerable side effects, and does not compromise any blinding of the studies. To perform studies in an outpatient clinic, it is essential to find a factor or compound which within short latency time induces the primary headache, in all its phases of premonitory symptoms, attack, and pain relief.
In this chapter we first consider human migraine models, an area that has been comparatively well studied. We then consider cluster headache (CH) models, some of which display similarities with certain migraine models. Finally, we describe the limited number of tension-type headache models currently available.
HUMAN MIGRAINE MODELS
Past experience has shown that besides the spontaneous attacks, migraine may be precipitated by a variety of factors, some of which are known to repeatedly induce a migraine attack. These have received interest as potential models of migraine (
65). Induction of migraine in healthy subjects is only rarely reported and then often in subjects with a family history of migraine. Thus, so far developing a migraine model only seems feasible in migraine patients. The proposed mechanisms for migraine induction are given as well as other actions of the compounds tested.
Table 23-1 shows an overview of the effects of several compounds tested for use as migraine models in migraine patients.
Glyceryl Trinitrate
The nitric oxide (NO) donor glyceryl trinitrate (GTN), which is used to treat angina pectoris, has proved to be an excellent compound for use in a migraine model and the model is well validated. From its use in cardiology, it is known to have well-described mechanisms of actions; well-known, mild, reversible, and short-lasting side effects, headache and hypotension being the most predominant; is easy to administer; and has a short half-life (
3).
GTN has long been known to induce headache as a side effect, and has over the years been proposed for use as a diagnostic test in migraine (
18,
71). The headache-initiating effects seem to be mediated through liberation of NO and not other metabolites (
38) (see
Chapters 15 and
30 for more details). In 1987, Sicuteri et al. described the biphasic headache response in migraine patients and in healthy subjects with a family history of migraine (
73). This study was followed up in several studies by the Copenhagen Group introducing GTN administration as a headache model using intravenous infusion of GTN in both healthy subjects, tension-type headache (TTH) and patients with migraine without aura (MO) (
36,
40,
63,
77).
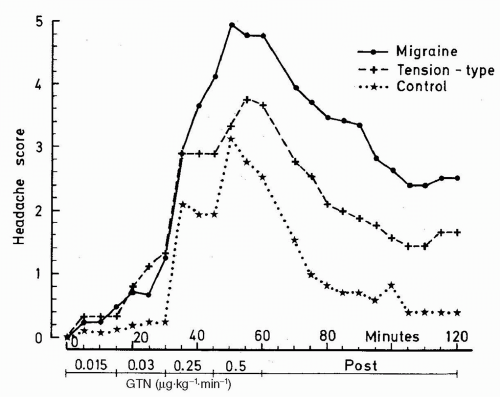
The GTN-induced headache consists of an immediate headache dissimilar to usual migraine. This develops in almost all patients and in a large group of healthy subjects with more intensive pain in MO patients than in both TTH and healthy controls (
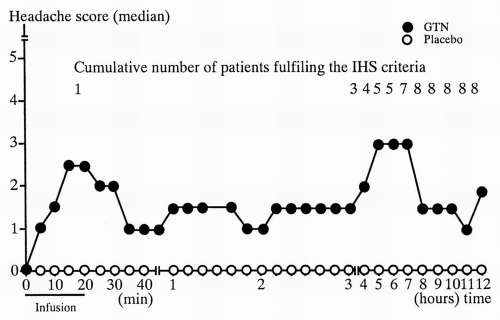
Fig. 23-1). In contrast to healthy subjects approximately 8 of 10 MO patients develop delayed headache resembling their usual MO attack with usual lateralization and accompanying symptoms fulfilling ICHD-1 criteria for MO, with peak headache score approximately 5.5 hours after infusion (
Fig. 23-2) (
77). Only very few healthy subjects experience a delayed headache. The basic studies showed good reproducibility and a dose-dependent headache response with a ceiling effect and maximum headache score at 0.5
μg · kg
−1 · min
−1 GTN; thus, this dose was applied in subsequent studies (see
Fig. 23-1) (
63).


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access






