High-Frequency Ventilation: Introduction
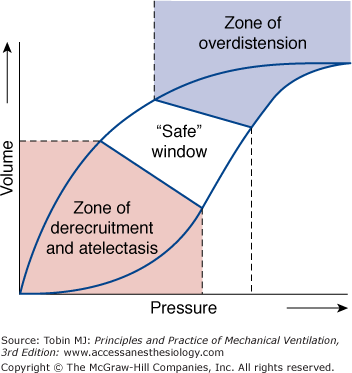
High-frequency ventilation has been an unconventional option for more than three decades and during that period several varieties of high-frequency ventilators have come and gone. Currently, interest in high-frequency ventilation in adult critical care is part of a larger search for ventilator strategies that can support gas exchange in the severely hypoxemic patient without contributing additional ventilator-induced lung injury. Over the past 30 years, high-frequency ventilators provided an experimental tool that identified many of the mechanisms that contribute to ventilator-induced lung injury. It became clear that ventilator-induced lung injury is minimized by ventilator patterns that achieve homogeneous aeration of as much of the lung as possible, avoiding both injury from overdistension (volutrauma) and that arising from the repetitive opening and closing of lung units in regions of atelectasis (atelectrauma) (Fig. 19-1).1 Failure to operate in the “safe zone” initiates biotrauma.2,3
Figure 19-1
Pressure-volume curve of a moderately diseased lung, as in a patient with acute lung injury. Ventilator-induced lung injury occurs at both extremes of lung volume. In the zone of overdistension, damage arises from edema fluid accumulation, surfactant degradation, and mechanical disruption. In the zone of derecruitment and atelectasis, lung injury arises from the direct trauma of repeated closure and reexpansion of small airways and alveoli, through accumulation and activation of inflammatory cells with release of cytokines (biotrauma), through interactions with local hypoxemia, by inhibition of surfactant, and through compensatory overexpansion of the rest of the lung as the lung “shrinks.” High end-expiratory pressures plus small tidal volume cycles are needed to stay in the safe window. (Reproduced, with permission, from Lippincott Williams & Wilkins, Froese AB, High-frequency oscillatory ventilation for adult respiratory distress syndrome: let’s get it right this time. Crit Care Med. 1997;25:906–908.)
The concept of a “safe zone” within which to ventilate the atelectasis-prone lung has been reflected in numerous clinical trials of lung-protective ventilation over the last 15 years. Conventional ventilator protocols have found survival benefit from shrinking the tidal volume and minimizing peak or plateau distending pressures.4 Studies such as that of Roupie et al5 and Terragni et al6 suggest that very small tidal volumes—even lower than 6 mL/kg predicted body weight—may be needed in some patients (those with the worst lung injury) to avoid overdistension. Very high levels of positive end-expiratory pressure (PEEP) would be needed in some patients to avoid derecruitment.7 Concurrently, high-frequency ventilation—both in oscillatory and jet forms—has become an established lung-protective modality in neonatal and pediatric intensive care (see Chapter 23).8–14 The question, however, persists: in severe acute respiratory distress syndrome in adult patients, will use of a high-frequency device result in clinically important outcome differences compared with lung-protective conventional ventilation, or are newer conventional ventilator protocols now equally able to protect the lung?15–17
Historical Overview
Several existing reviews detail the history of high-frequency ventilation.18,19 Early developments often were driven by issues peripheral to pulmonary critical care. Sjöstrand et al20 wanted to eliminate respiration-related variations in vascular pressures so that they could investigate carotid sinus reflexes and developed high-frequency positive-pressure ventilation. Lunkenheimer et al21 wanted to use the lungs as a route to deliver oscillatory pressure pulses to the myocardium. They needed apnea for this and were amazed to find gas exchange occurring while they applied their high-frequency flow oscillations. Emerson22 thought that high-frequency flow oscillations might provide internal physiotherapy and help to mobilize secretions. In Toronto, Bryan23 initially was curious to see whether an external “shaker” could enhance the gas mixing produced by cardiogenic oscillations. Klain and Smith24 explored jet ventilation at increasing frequencies to solve the problem of achieving alveolar ventilation in respiratory systems with a big leak, such as a bronchopleural fistula.
These devices often became intriguing phenomena in search of a reason for being. Devices such as high-frequency positive-pressure ventilation and high-frequency jet ventilation were particularly useful for surgical procedures when both anesthesiologist and surgeon needed access to the airway. The notion, however, that high rates and small tidal volumes might be of broader therapeutic value needed a pathophysiologic rationale.
An emerging concept in the 1970s was that many of the pulmonary perturbations that put patients into intensive care units were problems of low lung volume. Low lung volumes were associated with poor lung compliance, increasing airway and vascular resistances, increased work of breathing, airway closure, low ventilation–perfusion (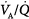 ) ratios, atelectasis, hypoxemia, and high oxygen (O2) exposure. Neonatal outcome was improving simply from the introduction of continuous positive airway pressure to improve alveolar aeration.25
) ratios, atelectasis, hypoxemia, and high oxygen (O2) exposure. Neonatal outcome was improving simply from the introduction of continuous positive airway pressure to improve alveolar aeration.25
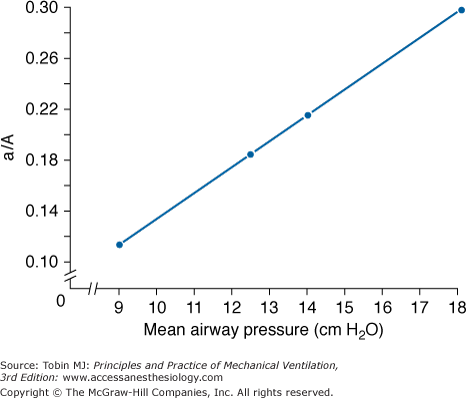
A crucial insight occurred early in our experience with high-frequency oscillatory ventilation (HFOV) when we explored a variety of mean airway pressure settings while ventilating an infant with neonatal respiratory distress syndrome (Fig. 19-2).26 The infant was stable in terms of both hemodynamics and CO2 elimination over the whole range of mean pressures tested, but oxygenation varied markedly. One could either ventilate with a low mean airway pressure (mPaw) and high fractional inspired oxygen concentration (FIO2) or a higher mean pressure and low FIO2. A choice had to be made. We gave priority to the reversal of low lung volumes. We argued that the small-volume cycles of HFOV should allow one to optimize alveolar aeration by using higher mean pressures than were considered safe during conventional mechanical ventilation while still keeping peak pressures less than those needed to eliminate CO2 at conventional rates. This pathophysiologic rationale continues to guide high-frequency applications to this day.
Figure 19-2
Relationship of oxygenation, as reflected in the ratio of the arterial and alveolar oxygen tensions (a/A ratio), to the mean airway pressure (mPaw) applied during HFOV, at constant tidal volume and frequency, in an infant with respiratory distress syndrome. No circulatory instability occurred over the entire range of mean pressures. CO2 elimination could be achieved equally well using a high FIO2 and a low mPaw or a low FIO2 and a higher mPaw. A choice of operating conditions had to be made. (From Marchak, et al. Treatment of RDS by high-frequency oscillatory ventilation: a preliminary report. J Pediatr. 1981;99:287–292. Reproduced with permission of Mosby, Inc.)
Many devices invented along the way, such as high-frequency positive-pressure ventilation, have since disappeared from use. High-frequency jet ventilation was explored in many adult intensive care units in the 1980s for difficult cases of bronchopleural fistula or tracheal disruption.27 It gradually became clear that any oxygenation benefits occurring during high-frequency jet ventilation resulted from increases in mean lung volume, not from some unusual properties of gas distribution.28 With an high-frequency jet ventilation device, the end-expiratory lung volume is a complex product of jet diameter and placement, driving pressure, jet frequency, and the time available for expiration.29,30 Safe use requires accurate intrapulmonary pressure monitoring, which was rarely provided with early devices. Inadvertent hyperinflation could cause problems with both circulatory depression and/or barotrauma. No North American, commercial, adult-sized jet ventilator was ever marketed with a safe, effective humidification system such as that available in Europe.
The largest early comparative trial of high-frequency jet ventilation versus conventional mechanical ventilation in hypoxemic lung disease was performed before the importance of atelectasis reversal was established. Consequently, it was carried out with the goal of supporting gas exchange with the lowest possible peak and mean pressures, and proved of no benefit.31 For all these reasons, high-frequency jet ventilation in adults has become a rare event. Jet ventilation at high or low frequencies continues to be a useful approach to situations in which airway access must be shared during surgical procedures32 or alveolar ventilation needs to be maintained in the presence of severe bronchopleural fistulas.29
High-frequency jet ventilation has persisted in neonatal and pediatric critical care largely because of the continuous design refinements of the Bunnell Life Pulse device.13,14 Early problems with poor humidification causing desiccation of tracheal mucosa were corrected, appropriate pressure-monitoring systems were devised, and expert training and technical support were provided.
A hybrid device combining high-frequency ventilation and conventional pressure-cycled ventilation (e.g., the high-frequency percussive ventilation/volume diffusive respirator [Percussionaire 4, Bird Technologies, Sandpoint, ID]) is used in many burn units.33 Its use was reviewed recently.34 Currently, the main high-frequency ventilatory options in the critical care of adults or larger children are HFOV or the high-frequency percussive ventilation/volume diffusive respirator Percussionaire. For neonates and small infants, high-frequency oscillatory ventilators, high-frequency jet ventilators, and the high-frequency percussive ventilator remain available. In view of HFOV’s increasing role in adult intensive care, this chapter focuses on HFOV.
Basic Principles of High-Frequency Oscillatory Ventilation
HFOV achieves gas transport with stroke volumes approximating anatomic dead space. Quasi-sinusoidal flow oscillations applied at the airway opening induce rapid gas mixing within the lungs. A number of physical transport mechanisms contribute to this mixing process. They have been reviewed in detail elsewhere.35
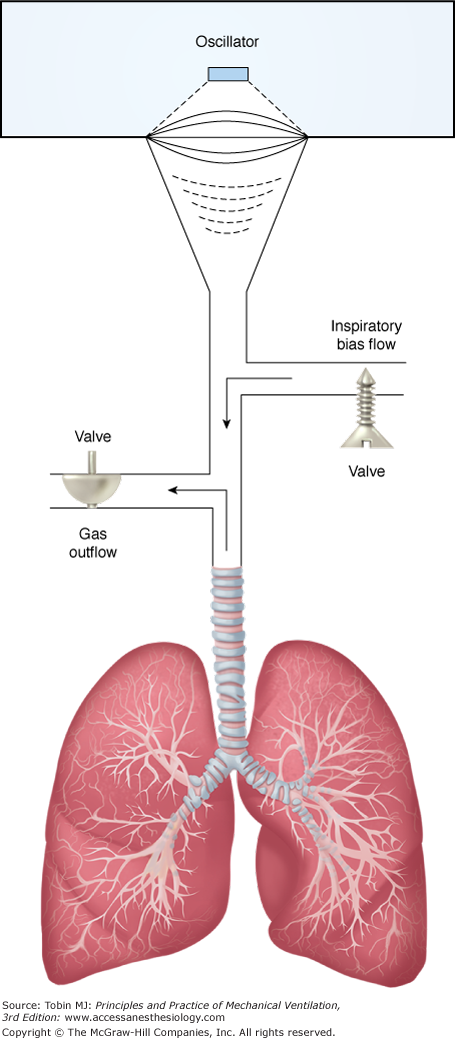
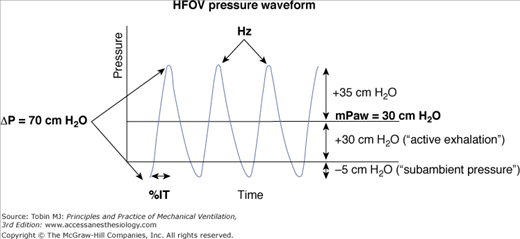
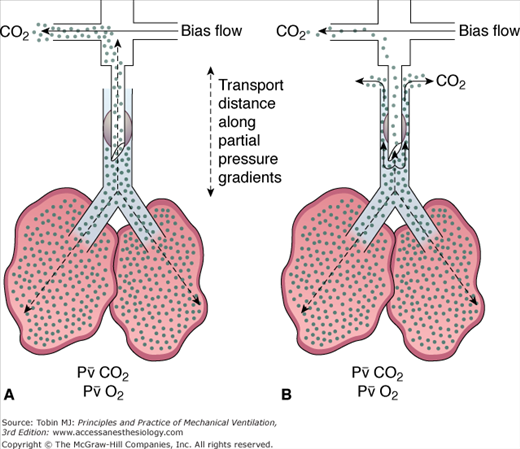
In practical terms, HFOV can be viewed as a mixing device that rapidly blends high O2/low CO2 gas from the top of the endotracheal tube with gas in the alveoli (Fig. 19-3). Net transport occurs along the partial-pressure gradients for O2 and CO2, with CO2 moving out of the lung along its partial-pressure gradient and O2 moving inward to the alveolar–capillary interface. These flow oscillations cause symmetric oscillations of intrapulmonary pressure (ΔP) around a mean distending airway pressure (mPaw) (Fig. 19-4). Although subambient pressures can occur in the circuit, intrapulmonary air trapping or “choke points” are unlikely with appropriate mPaw settings.36,37 One can view HFOV as a means of delivering “continuous positive airway pressure” with a built-in “shaker” to facilitate CO2 elimination.
Figure 19-3
Schematic of a circuit for delivery of HFOV. Quasi-sinusoidal flow oscillations are generated by a diaphragm or piston pump and directed to the endotracheal tube. A bias flow of humidified gas flushes the CO2 that is transported out of the lungs out of the circuit. Mean airway pressure is regulated by adjustments of the bias flow and the resistance of the expiratory limb. (From Krishnan, et al. High-frequency ventilation for acute lung injury and ARDS. Chest. 2000;118:795–807. Reproduced with the permission of the American College of Chest Physicians.)
Figure 19-4
Waveform of high-frequency pressure oscillations in the circuit above the endotracheal tube. Both inspiratory and expiratory flows are actively driven by the oscillator diaphragm. Pressure cycles equally above and below the mean level. When the oscillatory pressure amplitude (ΔP) is more than twice the mean airway pressure (mPaw), subambient pressures may occur in the circuit without inducing air trapping or choke points in the lung.37 The endotracheal tube filters the pressure swings, decreasing ΔP in the trachea and alveoli. Filtering is greater with smaller endotracheal tubes and higher frequencies.71,84,96 (From Derdak S. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adult patients. Crit Care Med. 2003;31:S317–S323. Reproduced with permission from Lippincott, Williams & Wilkins.)
In contrast to current conventional approaches to supplying PEEP with additional inspiratory assist for CO2 elimination, the mean distending pressure during HFOV is midway between the minimal and maximum values, introducing less risk of derecruitment during the expiratory phase for any given peak distending volume or pressure.38 One attraction of HFOV has been the way in which it uncouples the regulation of oxygenation and CO2 elimination into two separate control systems, unlike the situation with conventional ventilators, where it is often difficult to adjust one (i.e., the CO2 level) without also affecting the other. Oxygenation is regulated by reversing atelectasis and then finding the mean distending pressure that maintains alveolar expansion. The FIO2 then is set at a level that maintains appropriate arterial oxygenation goals. CO2 elimination is relatively independent of mean airway pressure,39 being regulated by frequency and stroke volume (i.e., power or ΔP) adjustments.40,41
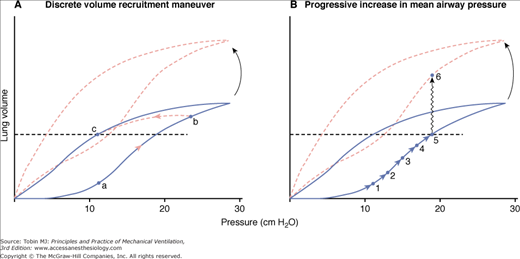
Although lung-volume optimization has become an accepted goal of HFOV, the “best way” to achieve this optimization remains controversial (Fig. 19-5). The small volume cycles of HFOV are simply not powerful enough to reopen atelectatic alveoli rapidly without some type of recruitment measure.
Figure 19-5
Schematic of two approaches to alveolar reexpansion during HFOV. The horizontal dashed line indicates the desired maintenance mean lung volume. The solid line is the pressure–volume relationship of a moderately diseased lung that still exhibits hysteresis. The dashed line indicates the pressure–volume relationship after some recovery has occurred. A. A brief sustained increase in mean airway pressure (mPaw) from a to b inflates the lung to near total lung capacity, putting it on the deflation limb of its pressure–volume curve. After this discrete recruitment maneuver, the target volume c is maintained at an mPaw of 11 cm H2O. If the pressure–volume relationship happens to change (as with position, diuresis, etc.), the operational lung volume remains constant. This is the approach used in the Treatment with Oscillation and an Open Lung Strategy (TOOLS) trial.54 B. A gradual march up the inflation limb of the pressure–volume relationship. Progressive increases in mPaw are used to achieve the target lung volume. An mPaw of 19 cm H2O is now needed to maintain lung volume at c. Also, if the pressure–volume relationship of the respiratory system changes, overdistension of the lung could occur (i.e., movement from point 5 to point 6). This is the approach used in the majority of neonatal and adult trials of HFOV to date. When actual lung volumes are measured, settings thought to have optimized lung volume clinically by these stepwise increases in mPaw often prove to be inadequate.61 (Froese AB. Neonatal and pediatric ventilation: Physiological and clinical perspectives. In: Marini JJ, Slutsky AS, eds. Physiological Basis of Ventilatory Support. 1998:1315–1357.)
All initial animal studies and one early human trial used a brief recruitment maneuver to near total lung capacity, followed by reduction of mPaw to a maintenance level that prevented derecruitment.42–46 This is termed getting the lung onto the deflation limb of its pressure–volume relationship. Recent investigations in both animal models and humans reinforce the value of ventilating on the deflation limb.47–54 After a recruitment maneuver, oxygenation goals are achieved at substantially lower maintenance mPaw values (near the point of maximum curvature),48,51,52 alveolar expansion becomes more homogeneous (which should reduce shear forces during ventilation and reduce volutrauma),50,55 and the percentage transmission of HFOV pressure cycles into the lung is decreased relative to settings producing equal shunt reduction on the inflation limb.49 Most studies in animals and humans have used one or more sustained inflation recruitment maneuvers to get the lung onto the deflation limb. A recent study in saline-lavaged pigs concluded that an escalating stepwise slow recruitment maneuver was more effective in opening the lung than brief sighs or a 20-second sustained inflation.56 This study, however, is limited by the low pressures employed and the incomplete recruitment seen with all techniques tested.
Following the High-Frequency Intervention (HIFI) trial of the late 1980s,57 fear of intraventricular hemorrhage in the fragile brain of the premature patient led most neonatologists to pursue stepwise increases in mPaw until either X-ray and blood-gas evidence of lung reexpansion was achieved or the mPaw level reached whatever level was deemed the “maximum allowable” level for a given institution.58 Physiologically, this can be described as “marching up the inflation limb” of the pressure–volume curve, as in Figure 19-5B. Most post-HIFI trial neonatal/pediatric trials (including the largest recent randomized, controlled trials in 200259,60) used stepwise increases in mPaw to “optimize” lung volume to oxygenation and chest X-ray targets. Unfortunately, when the actual lung volumes achieved by such clinical protocols are measured, many lungs prove to be suboptimally expanded.61 It follows that many comparative trials of HFOV and lung-protective conventional ventilation over the last 25 years have not achieved optimal homogeneous alveolar aeration at the lowest effective mean airway pressure, thus missing some of the potentially protective features of HFOV. A recent analysis of neonatal trials identified many design problems and stressed the need for further well-designed and monitored comparisons of ventilator options in this population.62 All early adult trials of HFOV started at mPaw levels of 3 to 5 cm H2O above the mPaw on conventional ventilation and then increased mPaw incrementally—again marching up the inflation limb.63–67
A few human trials have used protocols that rapidly place the lung “on the deflation limb” of its pressure–volume relationship.53,54 Although large comparative trials of the relative safety of these different recruitment protocols are not available, no evidence of risk from either barotrauma or intraventricular hemorrhage (IVH) has emerged using any of the neonatal or pediatric approaches since optimization of lung volume became an accepted goal of HFOV.8,9 What we do know is that lung-volume optimization is essential for HFOV to be protective of the lung, and alveolar aeration is more homogeneous and achieved at a lower mPaw after a recruitment maneuver, with or without use of the prone position, has moved the lung onto the deflation limb of the pressure–volume relationship. In a recent human trial, the oxygenation improvements gained in the prone position were only sustained when patients were managed with HFOV after being placed supine again.68 These observations suggest that the higher mPaw and small pressure and volume cycles of HFOV preserve alveolar reexpansion better than the current lung-protective tidal volumes of conventional ventilation.
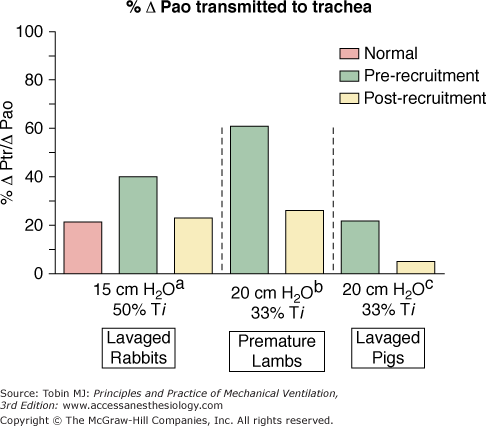
The relationship between the pressure swings at the airway opening (ΔPao) and those applied to the airways and alveoli (ΔPtr/ΔPao) is multifactorial. Atelectasis markedly impacts the percentage pressure transmission.69–72 Premature lambs,69 lavaged pigs,49 and lavaged rabbits72 all demonstrate the high cost of failure to reverse atelectasis, as reflected in the percentage of ΔPao transmitted to the trachea, bronchi, and alveoli (Fig. 19-6). Pressure swings and the risk of overdistension of aerated lung reduce rapidly to normal values following lung recruitment. Failure to reverse atelectasis during HFOV exposes airways and alveoli unnecessarily to potentially damaging shear forces. When van Genderingen et al49 analyzed intrapulmonary pressure transmission in terms of the ratio of intratracheal and circuit (machine displayed) ΔP values, termed the oscillatory pressure ratio, pressure transmission into the lung increased substantially at low lung volume. In vivo, adequate alveolar reexpansion optimized oxygenation while also minimizing intrapulmonary pressure swings. For a given level of shunt reduction, both the oscillatory pressure ratio and the mPaw needed to achieve optimal gas exchange were substantially lower after a recruitment maneuver.
Figure 19-6
Effect of a recruitment maneuver (RM) on the transmission of ΔP from the oscillator circuit (ΔPao) to a tracheal sampling site (ΔPtr). Alveolar reexpansion from an RM substantially reduced the amplitude of the peak-to-trough pressure swing applied to the trachea in lavaged rabbits,72 premature lambs,69 and lavaged pigs.49 Reversal of atelectasis protects lung tissue from potentially damaging shear forces. Note that ΔPmachine = ΔPcircuit = ΔPao in various sources.
Inadequate alveolar recruitment also may trigger inappropriate increases in ΔP or decreases in frequency because of hypercapnia, when what was really needed was alveolar reexpansion. All high-frequency oscillators currently available are load-dependent and deliver less tidal volume at any given setting when the lung is stiffer, as with ongoing atelectasis.73,74 If one’s goal is to explore HFOV for the potential lung-protective effects of maintaining O2 and CO2 transport at the smallest possible intrapulmonary pressure and volume swings, then lung-volume optimization is essential.
In the presence of recruitable lung, it costs less to pursue lung recruitment vigorously than to accept ongoing atelectasis.75 In late-phase disease with bronchiectasis, cysts, and progressive fibrosis, the opportunity for effective recruitment is past and should not be pursued. Similarly, in patients with milder acute lung injury (ratio of arterial partial pressure of oxygen to FIO2 is 200 to 300) whose lungs are already reasonably well aerated, attempts at further lung recruitment will likely only result in overdistension of the already open lung.76 All clinical trials done to date—from neonate to adult—that have mandated earlier lung-volume optimization have reported similar or decreased incidences of barotrauma, a transient need for inotropic support, and a decreased need for nitric oxide to reduce pulmonary vascular resistance despite using mean pressures in the thirties or forties when necessary to achieve oxygenation.11,64,77–80 Recent computed tomography studies during HFOV in eight adult patients with acute respiratory distress syndrome documented substantial (approximately 800 mL) increases in normally aerated volume with only a minor increase in hyperinflated lung (<50 mL).81 Overdistension certainly can occur, particularly if inappropriately high mPaw levels are maintained as the lung normalizes during treatment. Avoidance of this requires awareness and periodic reassessment of lung expansion.
Some algorithms warn against using mean distending pressures on HFOV higher than the plateau pressure limit of 30 cm H2O currently advocated for conventional ventilation. This warning may produce inadequate volume optimization, particularly in extrapulmonary acute respiratory distress syndrome. The risk of a given “maximum pressure” will vary with the size of the lung and the size and rate of delivery of the tidal volume engendering that plateau pressure. If 60% or 70% of the lung is not participating in gas exchange, then a tidal volume of even 6 mL/kg may induce dangerous overdistension of the ventilated alveolar units, as well as exerting shear forces on any alveolar units reexpanded during the course of the breath. If, instead, 70% or 80% of the lung is kept expanded throughout the ventilatory cycle, then a mean distending pressure of 30 cm H2O or more is safe, as proven in numerous clinical trials of HFOV,65,77,78,82 presumably because that distending pressure is now accommodated within many more participating lung units, fewer units are sheared open during the course of a cycle,38 and volume swings of only 1.5 to 2 mL/kg at high rates minimize peak distension. Conversely, if one fails to open the lung during HFOV and then keep it open, one will expose the lung to unwanted shear forces many more times per minute, particularly if forced to use lower rates and larger volume cycles because of inadequate recruitment.
Numerous studies have tried to predict an optimal maintenance mPaw from some feature of the static pressure–volume curve of the respiratory system, such as the inflection point on the inflation limb.51 These studies demonstrate a general property of the respiratory system, namely, that the mPaw needed to maintain a given level of oxygenation is substantially less after the lung has been inflated briefly to a pressure of 30 to 40 cm H2O so that it is “operating on the deflation limb.”49 Because recruitment and derecruitment pressures vary markedly between lung regions50,83 and between lung-injury models, generalizations to human ventilator management must be made cautiously. In clinical practice, a gradual deterioration in oxygenation over time that corrects with a recruitment maneuver indicates that the mPaw needs to be increased to keep alveoli/airways above their closing pressures. Conversely, a rise in the partial pressure of arterial carbon dioxide (PaCO2) at constant mPaw and power settings may indicate the lung is improving and is now overdistended, in which case a decrease in mPaw is needed.84 Nothing can replace thoughtful reassessment of both patient and machine factors.85 New bedside regional lung-volume monitoring techniques, such as electrical impedance tomography or respiratory inductance plethysmography, have the potential to clarify mPaw adjustments and may become more commonly used in the future.48,86–88
The basic transport studies of the 1980s were executed with accurate measurements of delivered stroke volumes over a wide range of frequencies using sinusoidal flow oscillations.40,89,90 They established that the volume of CO2 eliminated per minute (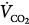 ) was proportional to the product of oscillatory frequency and the approximate square of the stroke volume (
) was proportional to the product of oscillatory frequency and the approximate square of the stroke volume (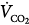 = VT∼2 · f). The endotracheal tube contributes a substantial impedance to gas transport during HFOV.91 If one uses an uncuffed endotracheal tube, or employs a partial cuff leak around a cuffed tube, such that the fresh-gas front moves to the bottom of the tube and CO2 exits around rather than through the tube, the stroke volume needed to achieve normocapnia decreases by up to 50% (Fig. 19-7). The bias gas-flow rate also influences transport efficiency through its effect on gas tensions at the fresh-gas front.92 Uncuffed tubes are used routinely in neonates and infants. Cuff leaks substantially assist CO2 elimination in adults,41,66 at the same time reducing intrapulmonary pressure swings.84
= VT∼2 · f). The endotracheal tube contributes a substantial impedance to gas transport during HFOV.91 If one uses an uncuffed endotracheal tube, or employs a partial cuff leak around a cuffed tube, such that the fresh-gas front moves to the bottom of the tube and CO2 exits around rather than through the tube, the stroke volume needed to achieve normocapnia decreases by up to 50% (Fig. 19-7). The bias gas-flow rate also influences transport efficiency through its effect on gas tensions at the fresh-gas front.92 Uncuffed tubes are used routinely in neonates and infants. Cuff leaks substantially assist CO2 elimination in adults,41,66 at the same time reducing intrapulmonary pressure swings.84
Figure 19-7
Schematic of the gas transport pathways during HFOV before and after establishment of a cuff leak. Transport distances are shown for CO2. On the left, with the endotracheal tube (ETT) cuff occluding the trachea, the fresh-gas front for both CO2 and O2 is at the top of the ETT. Gas transport mechanisms must bring CO2 from the alveoli to the top of the ETT before CO2 is flushed away by the bias flow. On the right, with partial ETT cuff deflation, some of the bias flow passes down the ETT and flushes CO2 molecules out beside the ETT, in effect reducing the transport distance substantially. The ETT represents 50% of the transport impedance during HFOV.71,91 Introduction of a cuff leak reduces the tidal volume needed to support CO2 elimination and decreases the intrapulmonary ΔP imposed on lung tissue.84 A cuff leak also may increase tracheal contamination by oral secretions. The risk-to-benefit ratio of a cuff leak is not yet established.
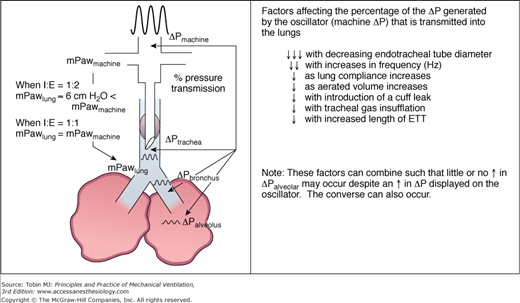
Early HFOV trials in neonates used a frequency of 15 Hz and an inspiration-to-expiration (I:E) ratio of 1:1. Demonstration of small interregional mean pressure gradients at 15 Hz and 50% inspiratory period (TI)93 subsequently induced a shift to a lower operating frequency of 10 Hz and a 33% TI in many centers. It is worth noting that although pressure gradients definitely occur in the lung during HFOV, their magnitudes are small (i.e., on the order of 1 to 3 cm H2O from apex to base).94 With a 33% inspiratory period, the mPaw measured in the circuit and displayed on the ventilator exceeds the mPaw within the lung by an amount that increases with tidal volume, frequency, and smaller endotracheal tube diameters, often in the range of 5 to 6 cm H2O (Fig. 19-8).95
Figure 19-8
Schematic of alterations in mPaw (left side of figure) between the oscillator circuit and lung using two different I:E ratios. Although mPaw values are relatively constant from the machine to the parenchyma with I:E = 1:1, mPaw decreases substantially along the endotracheal tube using an I:E = 1:2.73,95 On the right side of the figure the peak-to-trough pressure amplitude (ΔP on the machine) is markedly dampened by passage through the endotracheal tube and airways to the alveoli. Oscillatory waveforms are drawn to scale using data from Sakai.72 The associated table indicates multiple factors that reduce ΔP transmission into the lung.
Frequency selection directly affects the pressure cycles applied to the lung. A smaller percentage of the circuit ΔP is transmitted down an endotracheal tube at higher frequencies.71,96
Venegas and Fredberg97 approached the question of optimizing frequency during conventional ventilation as a problem of providing adequate alveolar ventilation at minimal pressure cost in a variety of clinical scenarios. Their approach provided graphic depictions of the shifting boundary conditions for safe ventilator settings with varying lung pathology. Although the normal lung can be ventilated over a wide range of frequencies and PEEP levels without inducing dangerous overdistension, in the lung with poor compliance but relatively normal airways PEEP selection becomes critical during conventional ventilation, with penalties in terms of pressure cost and overdistension with either too low or too high a level of PEEP. At high frequencies, the zone of safe PEEP widens, making it easier to maintain more of the lung homogeneously aerated. In lungs with increased airways resistance, the optimal frequency moves to a lower rate. The relationship between frequency and the pressure cost of alveolar ventilation has been developed further by Pillow for HFOV.70,71
Tidal volume delivery during HFOV is influenced by many factors (Table 19-1).26,98 Adult HFOV initially used frequencies much lower than those used in infants and most animal experiments because machine power appeared inadequate to achieve CO2 elimination at higher rates. Although the notion that HFOV delivers small tidal volumes has been challenged, newer data in adults suggest that very small tidal volumes can be delivered using adult HFOV, but at very low frequencies, tidal volumes may not be negligible.96,99 For optimal lung protection, theoretically one should use the smallest tidal volume and highest frequency that achieves PaCO2 elimination targets. Fessler et al100 studied thirty adult patients on HFOV after failing conventional lung-protective ventilation. CO2 elimination was adequate in 83% of them using mean maximal frequencies of 9.9 ± 2.1 Hz. Introduction of a cuff leak is recommended before decreasing frequency below 7 Hz.101 Even higher frequencies and small tidal volumes may prove preferable. The relative risk-to-benefit of early cuff leak versus lower frequencies requires further investigation.85,102 Resonant amplification of the delivered volume has been demonstrated,103,104 although at a median of 19 Hz in babies, well above rates used clinically.
|
Although inability to eliminate CO2 occurred in one of seventeen patients in the original report of Fort et al,77 the incidence of ventilation failure (i.e., pH ≤7.15 with bicarbonate ≥19 mEq/L) was the same in both HFOV and conventional ventilation groups in the subsequent larger Multicenter Oscillatory Ventilation for Acute Respiratory Distress Syndrome Trial (MOAT)82 and is rare if one follows current recommendations (see “Troubleshooting” below).
Physiologic Effects of High-Frequency Oscillatory Ventilation
Much like high levels of PEEP on conventional ventilation, HFOV is a sensitive detector of intravascular hypovolemia. Patients need intravascular volume repletion before one initiates HFOV and optimizes lung volume. Fortunately, clinical experience and animal studies demonstrate that despite this need for an adequate volume status, treatment with HFOV does not induce “wet lungs.”105 Alveolar expansion has a powerful impact on pulmonary vascular resistance. Pulmonary vascular resistance goes up both in areas of atelectasis and overdistension. Therefore, the impact of HFOV on pulmonary artery pressure will be influenced strongly by the extent to which homogeneous alveolar expansion can be achieved. This varies with lung pathophysiology, decisions made about mPaw and recruitment techniques, and body position. In clinical experience, pulmonary hypertension secondary to the use of higher mPaw during HFOV has not been a problem. Rather, HFOV often has enhanced the responsiveness to inhaled nitric oxide in both infants and adults.66,106–108 As with conventional lung-protective ventilator patterns, patients with preexisting right-ventricular failure need to be watched closely for the possible adverse effects of measures to optimize lung volume on right-ventricular output.
Cardiac output decreases as mPaw increases with any ventilator modality.109 In early HFOV trials in the premature baboon, cardiac output was less during HFOV45 unless mPaw was weaned as lung aeration improved.110 Similar interactions of cardiac output and mPaw occur in infants.111 Lesser hemodynamic impact can be expected when ventilating “on the deflation limb,” where alveolar expansion can be maintained at a lower mPaw cost.47,52 In adult clinical trials, such as the MOAT82 and TOOLS54 trials, there were no significant deleterious effects on mean arterial pressure, central venous pressure, pulmonary artery occlusion pressure, or cardiac output compared with conventional ventilation. Small (1 to 2 mm Hg), often transient increases in central venous pressure and pulmonary artery occlusion pressure were observed after transition to HFOV.67 Echocardiography has proven useful to assess preload adequacy when clarification is needed.