CHAPTER 22
Hepatitis
Julie Fishman, RPh, MPA
Hepatitis is an inflammation of the liver with hepatocellular damage ranging from mild to severe to potentially fatal. Hepatitis can be viral or autoimmune. Viral hepatitis is caused by six hepatotropic viruses: hepatitis A (HAV), B (HBV), C (HCV), D (HDV), E (HEV), and G (HGV). Autoimmune hepatitis (AIH) is a generally unresolving inflammation of the liver of unknown cause (Manns et al., 2010). The origin of hepatitis, the hepatitis virus type, and the degree of liver damage will determine the medical intervention needed. Patients with acute hepatitis should be monitored until liver function tests become normal. Acute cases of hepatitis due to known HCV exposure are additionally managed with antivirals. A severe acute case of AIH is treated with corticosteroids. Cases of known exposure to HAV and HEV should resolve quickly and spontaneously. Known exposure to HBV and HCV requires ongoing monitoring to detect and manage the chronic disease. The goal is to maximize the level of functioning by minimizing the severity of the liver failure.
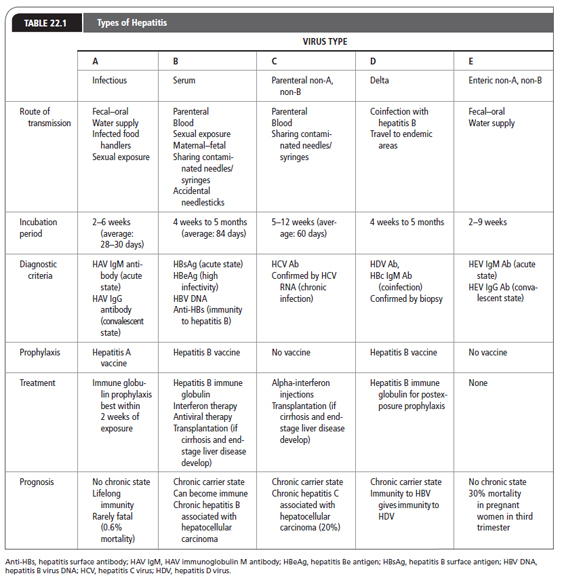
For purposes of this chapter, the hepatotropic viruses are reviewed (Table 22.1). Although HAV, HBV, and HCV are the viruses most commonly encountered in the United States, HDV, HEV, and HGV should be considered as well. Other viral causes of inflammation of the liver include adenovirus, Epstein–Barr virus, cytomegalovirus, rubella, herpes simplex, varicella, and parvovirus. Primary care providers must identify the virus causing liver damage, care for patients with these viral infections, and protect the community as well as themselves from exposure to these viruses.
 HEPATITIS A (INFECTIOUS HEPATITIS)
HEPATITIS A (INFECTIOUS HEPATITIS)
Anatomy, Physiology, and Pathology
HAV is a single-stranded RNA virus that belongs to the Hepatovirus genus in the Picoraniridae family. It is primarily transmitted by the fecal–oral route. Transmission is facilitated by poor sanitation, intimate contact (household or sexual), and poor personal hygiene. Every year, outbreaks from water or food contaminated by infected food handlers are reported.
Epidemiology
The Centers for Disease Control and Prevention (CDC) reported that there were 17,000 new cases of HAV in the United States in 2010. The annual number of new cases has decreased by 60% since 2005 (CDC, 2013d). HAV is a worldwide infection. It is nearly universal in childhood in overcrowded developing countries. It is thought that 29.1% to 33.5% of persons in the United States have been infected with HAV (CDC, 2013d). Risk factors for infection with HAV include employment at day-care centers, international travel, illicit intravenous drug use, exposure to contaminated food or water, men who have sex with men, people with blood clotting factor disorders, and personnel working with nonhuman primates (CDC, 2013a).
Diagnostic Criteria
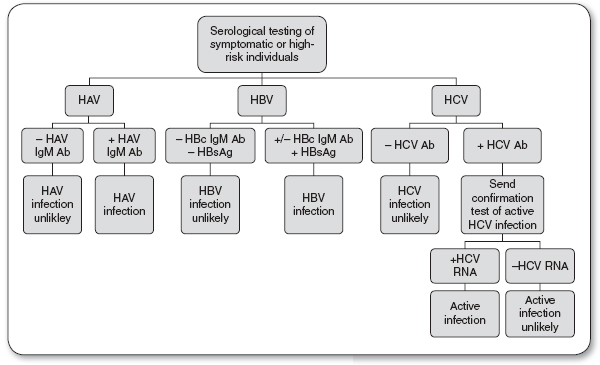
Serologic tests for screening and diagnosis of HAV include the HAV Immunoglobulin M antibody (HAV IgM Ab) and the HAV Immunoglobulin G antibody (HAV IgG Ab; see Figure 22.1).
 Presence of HAV IgM Ab indicates an acute HAV infection.
Presence of HAV IgM Ab indicates an acute HAV infection.
 Presence of HAV IgG Ab indicates a HAV convalescent state.
Presence of HAV IgG Ab indicates a HAV convalescent state.
History and Physical Examination
Symptoms consist of flu-like complaints. Jaundice may or may not appear. Other signs and symptoms of hepatitis can be found in Table 22.2. HAV is acute, self-limited, and rarely fulminant. Hepatic failure is rare, with a mortality rate of 0.5% (CDC, 2013b). There is no carrier state, and initial exposure confers lifelong immunity.
If these symptoms are vague in nature (e.g., fatigue, loss of appetite) they may not be sufficient to alert the patient to visit the primary care provider.
Persons infected with HAV may be asymptomatic and still have the potential to transmit disease. The incubation period is about 2 to 6 weeks (average: 28–30 days), with the highest concentration of HAV found during the 2 weeks before jaundice appears. It is during this period that the person is highly infectious. Although rare, HAV can be transmitted by blood transfusions if the donation is made in the prodromal phase of the infection.
Diagnostic Studies
Abnormalities may be present in the liver function test, but only liver enzyme alanine aminotransferase (ALT) is elevated before the onset of symptoms. The ratio of ALT to aspartate aminotransferase (AST) exceeds 1.4 (Salete de Paula, 2012). HAV is confirmed by finding HAV IgM Ab in the serum during the acute or early convalescent phase of illness. Immunocompromised patients and transplant recipients may have an acute HAV infection without the presence of HAV IgM Ab. In the rest of the infected population, this antibody to HAV usually declines to undetectable amounts within 6 months, and is rarely seen past 12 months. The liver enzyme tests return to normal before HAV IgM Ab becomes undetectable. HAV IgG Ab remains detectable in the serum for a lifetime and denotes a convalescent infection.
FIGURE 22.1
Diagnosis of acute hepatitis infection.
Ab, antibody; HAV, hepatitis A virus; HBV, hepatitis B virus; HBsAG, hepatitis B surface antigen; HCV, hepatitis C virus.
Source: Quest Diagnostics (2013).
Signs and Symptoms of Hepatitis |
During acute phases of any hepatitis infection, the patient may experience any or all of the following symptoms:
 Loss of appetite
Loss of appetite
 Nausea and vomiting
Nausea and vomiting
 Low-grade fever
Low-grade fever
 Malaise
Malaise
 Jaundice
Jaundice
 Change in color of urine or stool
Change in color of urine or stool
 Right upper quadrant pain
Right upper quadrant pain
 Liver enlargement
Liver enlargement
Treatment Options, Expected Outcomes, and Comprehensive Management
Prevention of HAV is enhanced by a clean water supply. This includes hand washing in restaurants and day-care centers. Use of cloth diapers would minimize the introduction of fecal matter into landfills from disposable diapers. Universal enteric precautions are recommended for health care providers, as they may care for potentially infectious asymptomatic patients. Outbreaks among health care workers show that inadequate hand washing and lack of appropriate glove use for handling stool result in exposure of health care workers to this virus.
HAV is a self-limiting disease. It does not progress to a chronic illness, but relapses have been observed. There currently is no treatment recommended for the disease itself, but symptomatic and supportive treatment should be given. These measures are discussed later in the section “Teaching and Self-Care.”
Prophylaxis
Hepatitis A vaccine is available and is given in two doses 6 to 12 months apart depending on the vaccine. The vaccine should be administered to children 1 year of age and to people in previously described risk categories. Vaccination offers greatest protection against HAV. Immune globulin can be administered before exposure when a patient is allergic to the vaccine, the vaccine is unavailable, or during the incubation period of HAV; it is most efficacious when given within 2 weeks of exposure. Immune globulin demonstrates up to 90% effectiveness postexposure (Liu, Nikolova, & Fei, 2010). Anyone with a known exposure to HAV should be tested for HAV infection and if not immune should receive the vaccine as well as prophylactic immune globulin. Known IgG to HAV means that the person has immunity to this infection and needs no prophylaxis. In cases of known exposure to HAV, close family members of the infected person should also be offered the hepatitis A vaccine, as well as immune globulin as prophylaxis, after they are tested for HAV antibodies. For travelers to countries where HAV is endemic, or for people who plan to be in close contact with natives of endemic countries, prophylactic administration of the hepatitis A vaccine plus a course of immune globulin before exposure is recommended (Sharapov & Teshale, 2013).
 HEPATITIS B (SERUM HEPATITIS)
HEPATITIS B (SERUM HEPATITIS)
Anatomy, Physiology, and Pathology
HBV is a DNA virus of the hepadnavirus family. It is transmitted parenterally, by sexual exposure, by contact with infected blood and tissues, and by maternal–fetal spread. HBV codes for a variety of proteins. There are three distinct antigens: surface antigen (HBsAg), core antigen (HBcAg), and the e antigen (HBeAg). These three antigens have antibodies that may appear during the infectious phase. There are eight genotypes of HBV (A through H), with genotypes A, B, C, and D being most common in the United States (Lok & McMahon, 2009).
Epidemiology
More than 2 billion people worldwide are infected with HBV, with 500,000 to 700,000 annual deaths. The highest incidence of HBV is seen in Africa (World Health Organization, 2012). In the United States, there are 800,000 to 1.4 million hepatitis B carriers (CDC, 2013d). There were about 38,000 new cases of HBV in the United States in 2010 (CDC, 2013d). HBV is prevalent in people born in countries where that virus is endemic, as well as their descendants. Most HBV-infected persons in the United States are intravenous drug users, men who have sex with men, and men and women with multiple sexual partners. Other at-risk groups are prison inmates, household contacts of HBV carriers, and infants born to HBV-infected mothers. Parenteral transmission can also occur from shared needles and tattooing. Health care workers are at risk through contact with blood and tissue. Voluntary screening of donated blood for hepatitis B antigens began in 1986. The current risk of HBV infection through blood transfusion is between 1 in 200,000 and 1 in 500,000 (American Red Cross, n.d.). The worldwide immunization of newborns and infants with the hepatitis B vaccine markedly decreased the incidence of HBV.
Diagnostic Criteria
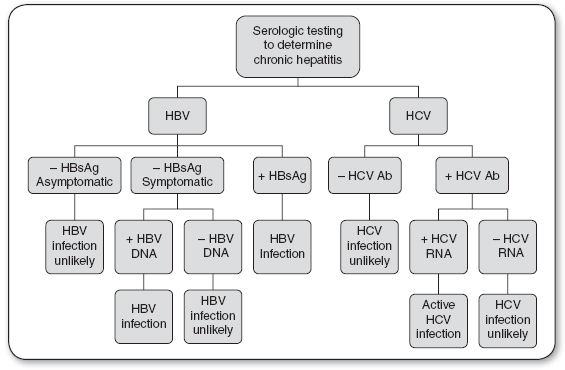
Serologic tests for screening and diagnosis of HBV include HBsAg, hepatitis B core antibody (HBcAb), and HBV DNA (see Figures 22.1 and 22.2).
 Positive HBsAg indicates an acute HBV infection.
Positive HBsAg indicates an acute HBV infection.
 Positive HBsAg six months after initial diagnosis indicates a carrier state or chronic HBV infection.
Positive HBsAg six months after initial diagnosis indicates a carrier state or chronic HBV infection.
 Negative HBsAg with the presence of clinical symptoms and a positive HBV DNA indicates chronic HBV infection.
Negative HBsAg with the presence of clinical symptoms and a positive HBV DNA indicates chronic HBV infection.
Clinical practice guidelines for hepatitis B from the American Association for the Study of Liver Disease (AASLD) can be found at www.aasld.org/practiceguidelines/Documents/Bookmarked%20Practice%20Guidelines/Chronic_Hep_B_Update_2009%208_24_2009.pdf
FIGURE 22.2
Diagnosis of chronic hepatitis infection.
Ab, antibody; HBV, hepatitis B virus; HBsAG, hepatitis B surface antigen; HCV, hepatitis C virus.
Source: Quest Diagnostics (2013).
History and Physical Examination
The incubation period of HBV is 4 weeks to 6 months, with an average of 84 days. During the acute phase, symptoms may last about 4 months. Typically, patients with acute HBV infections present with nonspecific complaints such as fatigue and anorexia. Jaundice may appear as the conjugated bilirubin level rises, and the patient may notice dark urine and clay-colored stool. Other signs and symptoms are noted in Table 22.2.
Less than 5% of adults infected with HBV, who are otherwise healthy, will become chronic carriers (World Health Organization, 2013). However, persons infected with HBV in infancy have an 80% to 90% likelihood of becoming chronic carriers in adulthood, and 15% to 25% of adults infected in childhood will die from cirrhosis or liver cancer (World Health Organization, 2013). The risk of being a HBV carrier is twofold: first, one can transmit the infection to others; second, one is at increased risk for the development of cirrhosis, liver failure, and primary liver cancer. Some chronic carriers are asymptomatic, whereas others experience symptoms that require intervention.
Diagnostic Studies
Nonspecific liver enzyme elevations (AST and ALT) may be the first indication that a patient has hepatitis. Clinical evidence of liver disease of at least 6 months’ duration, elevated serum aminotransferase levels with a liver biopsy showing an unresolved hepatic inflammation, and confirming serologic markers indicate chronic HBV infection. HBsAg appears in the blood about 1 month after exposure and may remain for up to 6 months. Persistence of HBsAg for more than 6 months indicates a carrier or chronic state. The presence of HBcAb occurs 2 weeks after HBsAg appears. The period between the disappearance of HBsAg and the appearance of hepatitis surface antibody (anti-HBs) is known as the window period. During the window period, the only serologic marker indicative of an acute HBV infection is the presence of HBc IgM Ab. The presence of anti-HBs indicates immunity to HBV.
Treatment Options, Expected Outcomes, and Comprehensive Management
There is no specific treatment for an acute HBV infection. Rather, supportive care must be provided based on the symptoms. The section titled “Teaching and Self-Care” at the end of this chapter outlines supportive care measures.
Treatment recommendations for chronic HBV carriers are based on three parameters: HBeAg presence, HBV DNA level, and ALT elevation. The treatment options include injectable pegilated interferon-α (PegINF) and oral drugs that are nucleoside analogue (NA) antiviral agents. These available therapies do not achieve cure. Therefore, the decision to initiate therapy is based on the risk of the liver-related morbidity and mortality, treatment safety and efficacy, possibility of drug resistance, and family planning for women.
The first-line medications recommended for treatment of HBV are PegINF and NAs (entecavir or tenofovir; Lok & McMahon, 2009). Other NAs used in chronic HBV are lamivudine, adefovir, and telbivudine. At least a 1-year course of NAs is recommended, with treatment continuing for at least 6 months after HBe antibody (HBeAb) seroconversion is achieved (Lok & McMahon, 2009). The most common side effects of PegINF therapy are neutropenia, which requires monitoring; and flu-like symptoms, which are transient and usually diminish with continued treatment. The NAs are generally well tolerated with a low incidence of side effects; however, patients should be monitored for renal toxicity. Dose adjustment is necessary for patients with a compromised creatinine clearance.
Active participation in the treatment plan is of utmost importance to prevent development of a resistant virus. Cross-resistance is common with this class of medication. If a patient develops breakthrough infection while on an NA, combining two NAs or making switches is recommended. HBV coinfection with HCV, HDV, or HIV may be encountered.
Liver transplant is the most effective option for HBV-infected patients, but the recurrence of HBV after liver transplantation is high. Prophylactic measures are imperative to prevent recurrence. These include hepatitis B immune globulin (HBIG) and antivirals. Without prophylaxis, the risk of HBV recurrence is 80%. This is principally related to the viral load at the time of the transplantation (Roque-Afonso, 2009). Most studies show that combining HBIG and lamivudine after liver transplant has demonstrated a <10% recurrence at 5 years (Roque-Afonso, 2009). Combination treatment recommendations with other antivirals are evolving.
Prophylaxis
There is a vaccine for HBV that is more than 90% effective (World Health Organization, n.d.[a]). It is a series of three injections, with the second dose given 1 month after the first, and the third dose given 6 months after the first dose. A fourth injection is recommended 6 months after the third dose in immunocompromised adults or when a faster immunogenic response is desired. Preexposure prophylaxis with immune globulin is not advised (World Health Organization, n.d.[b]). A combination of both vaccination and immune globulin is recommended after sexual and perinatal exposure.
The Occupational Safety and Health Administration (OSHA) mandates that health care workers be offered prophylactic vaccination if they expect to come in contact with blood or body fluids. This step, in combination with barrier protections, should minimize the risk of HBV transmission to health care workers. In the event of percutaneous or mucosal exposure, the treatment varies based on the vaccination and antibody response status of the exposed person and on the HBsAg status of the source of exposure. The specific guidelines can be found on OSHA’s website (www.osha.gov/OshDoc/Directive_pdf/CPL_2-2_69_APPE.pdf).
 HEPATITIS C (PARENTERALLY TRANSMITTED NON-A, NON-B HEPATITIS)
HEPATITIS C (PARENTERALLY TRANSMITTED NON-A, NON-B HEPATITIS)
Anatomy and Physiology
HCV was identified in 1989 as the causative agent in most cases of non-A, non-B hepatitis. HCV is a double-shelled, enveloped, single-stranded RNA virus classified in the Flaviviridae family and genus Hepacivirus (O’Shea, n.d.). There are at least six HCV genotypes based on the 5′ terminal region and nucleotide (NS5) sequence analysis. Genotype 1 (subtypes a and b) is most prevalent in the United States, followed by genotypes 2 and 3. The main route of transmission is parenteral, through sharing contaminated needles and syringes.
Epidemiology
HCV is the most prevalent of the hepatotropic viruses leading to chronic liver disease in the United States. In 2010, 17,000 new cases of HCV infection occurred in the United States, with the total number of infected people ranging from 2.7 to 3.9 million (CDC, 2013d). Approximately 70% to 85% of newly infected patients may develop chronic liver disease (Buggs, 2012). HCV is encountered worldwide, with the highest prevalence in Asia and Africa. HCV transmission is parenteral and can occur from the smallest exposure to infected blood. Risk factors include sharing needles and syringes, poorly sanitized tattoo and piercing equipment, and needlestick injuries among health care workers. HCV transmission through blood transfusion or blood products is uncommon because the screening of donated blood for HCV antigens began in 1990. The current risk of infection through blood transfusion is 1 in 1,390,000 (American Red Cross, n.d.). Transmission of HCV to household contacts of people with HCV infection is low. However, mother-to-child transmission has been documented through HCV RNA detection as a marker of infection in infants and occurs in 7% to 8% of HCV-infected mothers (Perinatology, 2013). Delivery by cesarean section does not prevent vertical transmission.
Diagnostic Criteria
Serologic tests for screening and diagnosis of HCV include the HCV antibody (HCV Ab) and HCV RNA (see Figures 22.1 and 22.2).
 Positive HCV Ab and HCV RNA indicate an acute or chronic HCV infection.
Positive HCV Ab and HCV RNA indicate an acute or chronic HCV infection.
 Negative HCV Ab and positive HCV RNA may indicate chronic HCV infection in an immunosuppressed patient, early acute HCV infection, or a false positive.
Negative HCV Ab and positive HCV RNA may indicate chronic HCV infection in an immunosuppressed patient, early acute HCV infection, or a false positive.

Full access? Get Clinical Tree