Chapter 62
Hemolytic Anemia
Clinical Clues to Hemolysis
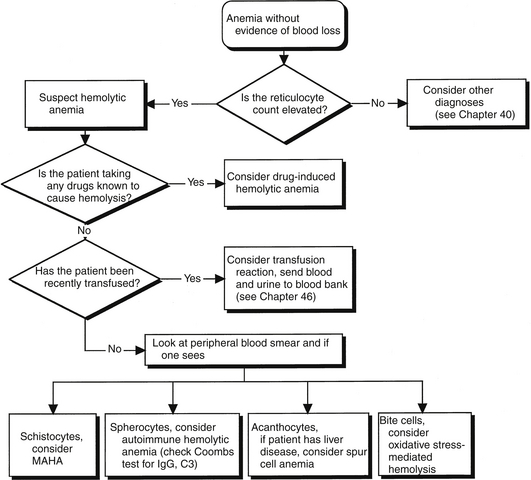
It is important to identify the clinical clues that indicate red blood cell destruction is taking place. Simply speaking, hemolysis can be defined as red cell destruction without evidence of frank blood loss. In general, hemolysis is accompanied by bone marrow compensation for a reduction in red cells. The most obvious example of a marrow response is an elevation in the reticulocyte count, which is generally observed whenever hemolysis is occurring. Other laboratory findings consistent with a diagnosis of hemolysis are an elevation in unconjugated bilirubin, an elevation in the level of lactate dehydrogenase, hemoglobinemia, hemoglobinuria, and a reduction in the serum haptoglobin concentration. These findings are consistent with, but not diagnostic of, hemolysis because these tests have relatively low sensitivity and specificity. As with other hematologic disorders, a review of the peripheral blood smear is essential for making the diagnosis of hemolytic anemia. Findings on the peripheral smear can identify specific pathophysiologic processes (Figure 62.1).
Hemolysis can be divided into two major groups: those that are inherited and those that are acquired (Box 62.1).
Inherited Hemolytic Anemias
Red Blood Cell Enzyme Disorders
G6PD deficiency has its highest prevalence among Kurdish Jews, with an estimated prevalence of more than 60%. G6PD deficiency is also found in malaria-rich regions of the world such as Africa. As the gene coding for G6PD is on the X chromosome, males are preferentially affected. G6PD-deficient individuals, as a rule, do not have significant hemolysis unless subjected to oxidant stress, which is usually caused by drugs or fever. Hemolysis is most often secondary to exposure to oxidant drugs, such as quinine, phenazopyridine, or dapsone. A more complete list of medications to be avoided in G6PD-deficient patients can be found in Box 62.2.
Diagnosis of G6PD deficiency can be made by review of the peripheral smear that may show red blood cell blistering or Heinz bodies (inclusions of denatured hemoglobin seen in smears stained with methylene blue). Laboratory analysis of G6PD levels and phenotype should be performed at times other than acute hemolytic episodes because the cells that remain after a hemolytic episode tend to be those with higher levels of G6PD. Proper treatment of this disorder is avoidance of exposure to these oxidant drugs. Once hemolysis occurs, it tends to be self-limited and the care of patients remains supportive, including judicious use of red cell transfusions only when hemolysis is of crisis proportion.
Globin Chain Production and Structure Disorders
Disorders of globin chain production and structure include the thalassemias and hemoglobinopathies, such as hemoglobin S and hemoglobin C. These mutations cause unstable hemoglobins, which shorten red blood cell survival. Of relevance to ICU care, patients with sickle cell anemia can develop acute chest syndrome characterized by shortness of breath, chest pain, hypoxia, fever, and an infiltrate on chest radiograph. These patients may become critically ill and may require mechanical ventilation. Acute chest syndrome may be secondary to in situ thrombosis, pneumonia, or fat embolism. Regardless of etiology, patients with acute chest syndrome require red cell replacement either through simple transfusion or exchange transfusion. Red cell replacement is associated with improvement in morbidity and mortality. When exchange transfusions are required, the goal is a hemoglobin of 10 g/dL with 30% sickled cells.
Acquired Hemolytic Anemia
Acquired hemolytic anemias can be severe and can result in ICU admission (Table 62.1).
Table 62.1
Treatment of Acquired Hemolytic Anemias
| Disorder | Treatment |
| Paroxysmal cold hemoglobinuria | Usually self-limited; can treat underlying disease |
| Warm antibody | Prednisone; may also need danazol, IgG, or splenectomy; rituximab (rituxan) for patients refractory to steroids; immune globulin and splenectomy |
| Cold agglutinin disease | Keep patient warm; prednisone not helpful |
| Drug induced | Remove offending agent |
| Acute hemolytic reaction | Immediately stop transfusion; support blood pressure, control bleeding, and maintain urine output |
| Microangiopathic hemolytic anemia | Treat underlying condition; avoid giving platelets |
| Infectious causes | Treat underlying infection |
| Spur cell anemia | Supportive care |
| Paroxysmal nocturnal hemoglobinuria (PNH) | Usually supportive (blood transfusion, anticoagulation) eculizumab (Soliris) in select patients |

Full access? Get Clinical Tree