Key Clinical Questions
Reduced Ejection Fraction
A 66-year-old man presents with progressive history of breathlessness, decrease in activity tolerance, orthopnea and paroxysmal nocturnal dyspnea. His past medical history includes diabetes and a myocardial infarction 5 years ago. On physical examination the patient appears dyspneic with a blood pressure of 145/90 and a heart rate of 100 beats per minute (bpm). The jugular venous pressure is 14 cm H2O, the point of maximum impulse is displaced laterally, and the heart sounds are distant with an S3 gallop. There are rales in the lung bases with abdominal right upper quadrant fullness; the lower extremities are cold with significant pitting edema. |
Heart failure (HF) is a condition that affects millions in the United States, and over half a million people are newly diagnosed each year. The incidence of HF increases with age, affecting 6% to 10% of people age older than 65. It is the leading cause of hospital admissions in the United States and is responsible for 6.5 million hospital-days each year and up to 15 million office visits. Over the past decade, the rate of hospitalizations for HF has increased by 159%, and 45% of patients hospitalized with acute heart failure will be rehospitalized at least once (and 15% at least twice) within 12 months.
Approximately 260,000 patients die of HF in the United States each year. The number of HF deaths has increased steadily despite advances in treatment, in part because of aging of the population and improved survival after acute myocardial infarction (MI). The estimated direct and indirect cost of HF is $27.9 billion per year, and approximately $2.9 billion annually is spent on drugs for the treatment of HF.
Heart failure has been identified by The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) as a priority focus for hospital core measure development. The four HF core measures are
- HF-1—Discharge instructions
- HF-2—Evaluation of left ventricular systolic function
- HF-3—Angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) for left ventricular systolic dysfunction
- HF-4—Adult smoking cessation advice/counseling.
Heart failure is a complex clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill or eject blood and maintain metabolic demands of the body. It is characterized by specific signs and symptoms, such as dyspnea, fatigue which may limit exercise tolerance, and fluid retention which leads to pulmonary and peripheral edema.
Heart failure may result from disorders of the pericardium, heart valves, myocardium, or coronary circulation as well as rhythm disturbances. The majority of patients have symptoms due to an impairment of left ventricular (LV) myocardial function, with or without a reduced ejection fraction. The etiologies for LV dysfunction are broad, coronary artery disease being the leading cause in developed countries (Table 130-1).
| Coronary artery disease |
| Idiopathic dilated cardiomyopathy |
| Familial cardiomyopathy |
| Neuromuscular disorders (eg, muscular dystrophies) |
| Chagas cardiomyopathy |
| Toxins (eg, alcohol, cocaine) |
| Medications (eg, adriamycin) |
| Diabetes mellitus |
| Myocarditis (lymphocytic, hypersensitivity, giant cell) |
| Infiltrative (amyloidosis, sarcoidosis, hemochromatosis) |
| Metabolic disorders (hypothyroidism) |
| Peripartum |
| Untreated valvular heart disease (aortic stenosis or regurgitation, mitral regurgitation) |
| Tachyarrhythmias (atrial and/or ventricular arrhythmias) |
| Autoimmune disorders (lupus, rheumatoid arthritis) |
| Hypertension |
Heart failure is generally a progressive disorder that results after an insult or stress to the myocardium resulting in cardiac remodeling (eg, ventricular enlargement and hypertrophy) with myocyte hypertrophy and apoptosis, myocardial fibrosis, and changes in calcium cycling. These processes increase hemodynamic wall stress causing a further decline in myocardial mechanical performance; ventricular enlargement leads to incompetence of the mitral valve and regurgitation. Mitral regurgitation leads to decreased forward stroke volume, elevated pulmonary venous pressure, increased wall stress and ultimately more remodeling. In response to progressive cardiac dysfunction, neurohormonal systems (in particular the renin-angiotensin-aldosterone and adrenergic systems) become activated to provide acute hemodynamic stabilization. Patients with HF have elevated circulating or tissue levels of norepinephrine, angiotensin II, aldosterone, endothelin, vasopressin, and cytokines. However, when chronically stimulated, these systems act to adversely affect the structure and function of the heart. Neurohormonal activation increases the hemodynamic stresses on the ventricle by causing sodium retention and peripheral vasoconstriction that subsequently lead to further remodeling and more neurohormonal stimulation. The remodeling process precedes the development of symptoms, and in conjunction with the activation of the neurohormonal cascade, continues after the appearance of symptoms and contributes to worsening of symptoms, development of arrhythmias, and ultimate mortality despite optimal therapy.
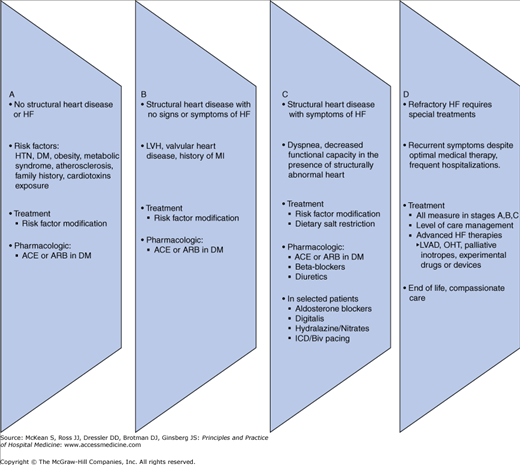
The 2005 ACC/AHA task force developed a new classification scheme outlining four stages in the evolution of the HF syndrome (Figure 130-1), recognizing established risk factors, structural prerequisites, asymptomatic and symptomatic phases, and appropriate therapeutic interventions at each stage to reduce morbidity and mortality.
Figure 130-1
Stages in the development of heart failure/recommended therapy by stage. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; EF, ejection fraction; FHx CM, family history of cardiomyopathy; HF, heart failure; LV, left ventricular; LVH, left ventricular hypertrophy; and MI, myocardial infarction.
There are two states of heart failure, compensated and acute decompensated heart failure (formerly “congestive heart failure”). Heart failure is predominantly characterized by an elevation in left atrial and subsequently pulmonary venous and arterial pressures. The net result may be transudation of excess fluid into the alveolar spaces, leading to decreased diffusing capacity, hypoxia, and shortness of breath. Inadequate cardiac output may also complicate the hemodynamic and clinical picture and generally represents a poor prognostic feature.
Clinical manifestations vary among individuals. The chronicity, severity and degree of hemodynamic decompensation as well as patient specific factors such as age, gender, and other comorbidities influence symptoms. Clinical signs and symptoms of HF lack sensitivity and specificity. Exercise intolerance, usually a predominant symptom, but does not correlate with the degree of LV dysfunction as assessed by ejection fraction. The New York Heart Association (NYHA) functional classification is used to assess exercise tolerance and should be determined in the ambulatory and compensated state.
- I = No symptoms
- II = Symptoms with moderate or marked levels of activity
- III = Symptoms with mild activity
- IV = Symptoms at rest
Elevated left atrial and pulmonary capillary wedge pressures are responsible for “congestive” symptoms such as shortness of breath, cough, orthopnea, paroxysmal nocturnal dyspnea, and peripheral edema. Early satiety, right upper quadrant tenderness, and anorexia may be subtle signs of volume overload. A reduced cardiac output may be manifest as easy fatigability, mental status changes and/or a decrease in exercise tolerance. Angina may be present in both ischemic and nonischemic cardiomyopathies.
A careful physical examination is the cornerstone of the evaluation of patients with heart failure. The examination helps to determine the status and severity of the hemodynamic profile which then guides a therapeutic plan. The examination should focus on determining the hemodynamic picture along two axes: degree of volume overload (“wet” or “dry”) and adequacy of perfusion (“warm” or “cold”). Findings consistent with elevated cardiac filling pressures and fluid overload include elevated jugular venous pressure, hepatojugular reflux, gallops (S3 and S4), murmurs of tricuspid or mitral regurgitation, pulmonary rales, hepatomegaly, ascites, and edema. Narrow pulse pressure, cool extremities, altered mentation, Cheyne-Stokes respiration, and a resting tachycardia suggest a marked reduction in cardiac output. Hypotension may indicate severe ventricular dysfunction and impending cardiogenic shock. The presence of peripheral edema and pulmonary rales, although relatively specific, are not sensitive and may be absent. The etiology of cardiac dysfunction may also be apparent at the time of examination although the differential diagnosis is broad (Table 130-2). A comprehensive evaluation should be performed.
| Myocardial ischemia |
| Pulmonary disease (pneumonia, asthma, chronic obstructive pulmonary disease, pulmonary embolus, pulmonary arterial hypertension) |
| Sleep disordered breathing |
| Obesity |
| Malnutrition |
| Hepatic failure |
| Renal failure |
| Hypoalbuminemia |
| Venous insufficiency |
| Depression and deconditioning |
| Pericardial disease |
Echocardiography is critical to the determination of etiology, particularly distinguishing those patients with predominantly systolic heart failure from those with valvular heart disease or preserved ejection fraction (eg, > 50%). Electrocardiogram, full laboratory assessment, and a chest x-ray should be performed in all patients presenting for the first time with heart failure. Additional testing (eg, right heart catheterization, brain natriuretic peptide [BNP] or N-terminal proBNP) should be considered but individualized.
The goal of the initial triage is to establish a cause for decompensation (Table 130-3) as well as defining the hemodynamic picture using the physical examination and history. As noted above, the hemodynamic picture can be described as one of four states on the basis of two axes: volume status (overload [“wet”] or euvolemic [“dry”]) and perfusion or cardiac output (well perfused [“warm”] or low output [“cold”]) in order to place the patient in one of four categories (Figure 130-2).
| Acute coronary syndrome |
| Uncontrolled hypertension |
| Acute arrhythmia (ventricular or supraventricular) |
| Valvular heart disease |
| Acute severe myocarditis |
| Medical or dietary noncompliance |
| Infections |
| Perioperative volume resuscitation |
| Drug or alcohol abuse |
Acute decompensated heart failure, whether in the context of new onset or chronic, requires urgent treatment and should focus on hemodynamic stabilization, oxygenation and organ perfusion, and restoring filling pressure to optimal levels.
Initial laboratory evaluation of patients with HF should include complete blood count, urinalysis, serum electrolytes (including calcium and magnesium), blood urea nitrogen, serum creatinine, fasting blood glucose, liver function tests, lipid profile, thyroid function tests, and cardiac biomarkers. Screening for sleep-disordered breathing, HIV, rheumatologic disorders, and pheochromocytoma are reasonable in selected situations.
Twelve-lead electrocardiogram should be obtained to assess for rhythm, conduction system abnormalities and rule out acute coronary syndromes. Chest x-ray is useful in identifying cardiomegaly, pulmonary congestion, and concomitant pulmonary disorders such as pneumonia or chronic obstructive pulmonary disease.
Echocardiography is critical in the initial evaluation of patients with new onset HF to obtain information on left ventricular wall thickness, end diastolic and end systolic dimensions, right ventricular function, ejection fraction, valvular and pericardial disease. Serial assessments may be useful after optimizing medical and device therapy or a change in clinical status. Coronary angiography should be performed in patients presenting with HF who have angina or significant ischemia unless they are not candidates for revascularization. Endomyocardial biopsy is reserved for patients in whom a specific diagnosis is suspected (eg, hemochromatosis, amyloidosis, sarcoidosis) if it would change management. Brain natriuretic peptide or N-terminal pro-BNP (secreted by the cardiac ventricles in response to wall stress) can be useful in the urgent care setting when the diagnosis in patients presenting with dyspnea is uncertain. Both markers have good negative predictive values to exclude HF and can provide prognostic information if HF is confirmed. Their use to guide therapy has not yet been established.
A risk stratification tool developed from the Acute Decompensated Heart Failure National Registry (ADHERE) identified the following variables as predicting higher risk for in-hospital mortality:
- Blood urea nitrogen > 43 mg/dL
- Admission systolic blood pressure < 115 mm Hg
- Serum creatinine > 2.75 mg/dL
Other parameters that have been correlated with clinical outcomes in patients hospitalized with HF include age, heart failure etiology (ischemic cardiomyopathy has poorer prognosis), anemia, hyponatremia, B-type natriuretic peptide levels, and left ventricular ejection fraction (LVEF).
The management of acute decompensated heart failure (ADHF) can be divided into two aspects. The acute management is directed toward hemodynamic recovery to improve symptoms and identification of complicating comorbidities; chronic treatment is directed at preserving functional capacity, preventing rehospitalizations, and improving long-term survival.
The initial phase of management is to identify and target one of the four hemodynamic profiles as previously described. It should be recognized that the vast majority of patients presenting with acute decompensated heart failure will be volume overloaded yet adequately perfused (so-called “wet and warm”). The relief of congestion is therefore a predominant goal of acute therapy.
Intravenous loop diuretics such as the most commonly used, furosemide, will produce a diuresis accompanied by a prompt drop in preload and relief of symptoms related to pulmonary congestion (Table 130-4). Intravenous loop diuretics may also exert a vasodilating effect.
| Drug | Initial Dose | Maximum Daily Doses |
|---|---|---|
| Loop diuretics | ||
| Bumetanide | 1 mg | 5–10 mg |
| Torsemide | 20 mg | 100–200 mg |
| Furosemide | 40 mg | 160–320 mg |
| Thiazide diuretics (given 30 min before loop diuretic to augment diuresis) | ||
| Hydrochlorothiazide | 25 mg | 100 mg |
| Chlorothiazide | 250–500 mg IV once or twice daily plus loop diuretic | |
| Metolazone | 2.5–10 mg orally once or twice daily with loop diuretic | |
| IV continuous infusion | ||
| Bumetanide | 1 mg IV load then 0.5–1 mg/h | |
| Torsemide | 20 mg IV load then 5–10 mg/h | |
| Furosemide | 20–80 mg IV load then 5–20 mg/h |
The peak diuretic effect typically occurs 30 minutes after administration and should be titrated to response. The administration of a thiazide diuretic, such as metolazone, 30 minutes prior to a dose of furosemide can potentiate its effect. Continuous infusions may be considered although they appear to be comparable to properly dosed bolus treatment. Overly rapid diuresis with an excessively rapid reduction in intravascular volume may result in symptomatic hypotension and/or worsening renal function.
Morphine may be useful because it induces venodilation and mild arterial dilatation and relieves breathlessness and anxiety.
Supplemental oxygen should be initiated and pulse oximetry monitored. Noninvasive positive pressure ventilation, if necessary for acute respiratory failure, is associated with a significant reduction in the need for tracheal intubation and mechanical ventilation.
Vasodilator therapy should be initiated in the absence of symptomatic hypotension. Short-acting ACE inhibitors, ARBs, or hydralazine are the drugs of choice in most situations. Intravenous vasodilator therapy may be considered if rapid improvement of congestive symptoms is needed but requires close hemodynamic monitoring, ideally in an ICU setting.
Nitrates decrease systemic and pulmonary vascular pressures and are also coronary vasodilators.
Nitroprusside is indicated when there is a need for acute combined afterload and preload reduction (hypertensive emergency, acute aortic regurgitation, acute mitral regurgitation).
Nesiritide has venous, arterial, and modest coronary vasodilatory properties that reduce preload and afterload and has no direct inotropic effects. It is currently only approved for the acute relief of dyspnea in the patient with decompensated heart failure. Because of its hypotensive effects, it should be used only at recommended doses and with caution.
Intravenous inotropic agents (dobutamine, milrinone, dopamine) are indicated in the presence of peripheral hypoperfusion (hypotension, decreased renal function) with or without pulmonary edema refractory to the above treatment modalities. The benefit of hemodynamic improvement may outweigh the risk of arrhythmias and increase in oxygen demand caused by these agents. These patients are most appropriately managed in cardiac care units. These agents should only be used for short-term circulatory support and should not be used routinely for management of acute decompensated heart failure.

Full access? Get Clinical Tree