Chapter 14
Health Care–Associated Infections
The Centers for Disease Control and Prevention (CDC) estimates that 1 out of every 20 patients hospitalized in an acute-care hospital in the United States develops a health care–associated (nosocomial) infection (HAI), accounting for up to $45 billion in direct costs. HAIs occur 5 to 10 times more often in intensive care units (ICUs) and significantly increase morbidity, mortality, and length of hospital stay. This chapter describes the prevention, diagnosis, and treatment of the four most common HAIs in ICUs: (1) catheter-related bloodstream infections (CRBSIs), (2) ventilator-associated pneumonias (VAPs), (3) catheter-associated urinary tract infections (CAUTIs), and (4) surgical site infections (SSIs). Table 14.1 lists commonly associated organisms with these infections.
TABLE 14.1
Sites of Intensive Care Unit Nosocomial Infections and Commonly Associated Pathogens
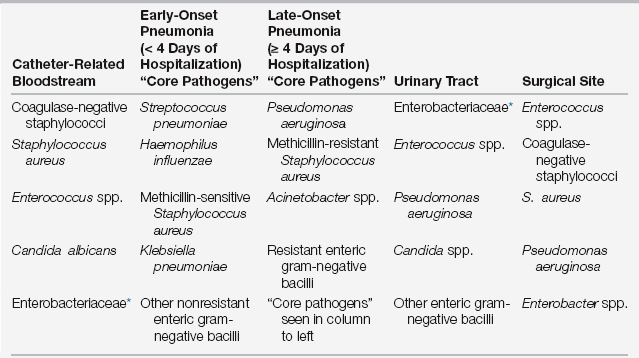
∗Enterobacteriaceae includes more than 70 genera of gram-negative bacilli, with Escherichia coli, Klebsiella species, and Enterobacter species most common in healthcare-associated infections.
Approach to Infection Control in the Intensive Care Unit
Infection Control Policies
The department of infection prevention and control should provide quantitative infection data to the ICU leadership team and staff so that HAIs can be investigated and appropriate prevention and quality improvement measures initiated.
Special Infection Risks in the Intensive Care Unit
Broad-spectrum empiric antibiotic use is also more common in ICUs (Chapter 18), predisposing patients to infection or superinfection with fungal organisms, multidrug-resistant organisms (MDROs), and Clostridium difficile. To minimize these risks, the need for antibiotics should be carefully evaluated prior to their initiation. If antibiotics are necessary, the narrowest effective antibiotic regimen should be selected based on most likely organisms. However, before initiating empirical antimicrobial therapy, all appropriate cultures based on suspected sources of infection should be obtained so that the antibiotic regimen can be tailored to the cultures and susceptibility results. The continuation of empiric antimicrobial therapy is an issue that should be addressed daily on rounds and should be justified by culture results and the patient’s clinical status (Chapter 18). Judicious antibiotic use should be viewed as a requisite for effective infection prevention and control.
Infections Due to Intravascular Catheters
Clinical Definitions and Surveillance Definitions
A CRBSI is an infection of an intravascular catheter or catheter site with associated bacteremia. As with other device-associated infections in the ICU, it is important to understand the difference between clinical definitions (those used by clinicians to make a diagnosis and guide management) and surveillance definitions (those used by infection prevention and control personnel to monitor and report HAIs). Each definition has different objectives and has different criteria designed to achieve these goals. Therefore, a particular case may meet the surveillance definition but may not meet the clinical definition or vice versa.
Incidence Rates and Pathogens
The overall incidence of CLABSIs reported by NHSN is between 1.3 and 5.6/1000 catheter days (1 catheter day = 1 catheter in 1 patient for 1 day). Estimates are that the number of CLABSIs originating in ICUs has decreased by 58% from 2001 to 2009 at least in part because of an increased national focus on infection prevention and control. However, certain characteristics of ICU patients place them at higher risk (Box 14.1). Most CRBSIs are secondary to gram-positive organisms (see Table 14.1). Factors that increase the likelihood of gram-negative CRBSI include critical illness, neutropenia, prior antibiotic exposure, and femoral site. Factors that increase the likelihood of Candida CRBSI include parenteral nutrition, prolonged exposure to broad-spectrum antibiotics, hematologic malignancy, organ or bone marrow transplantation, femoral site, and presence of multiple sites of Candida colonization elsewhere in the patient (urine, respiratory, etc.).
As with other infections in the ICU, antibiotic resistance is a problem. Methicillin-resistant S. aureus (MRSA) represents over 50% of isolates of S. aureus in some regions. Vancomycin-resistant Enterococcus (VRE) is also becoming a more prevalent cause of CRBSI. Emergence of extended-spectrum beta-lactamases (ESBLs) and carbapenemases in gram-negative organisms has resulted in resistance to most available antibiotics. There is increasing resistance to fluconazole among non-albicans Candida species, and fluconazole resistance has also been reported in C. albicans.
Prevention
Efforts at prevention of CLABSI have yielded impressive and sustained results, including reports from many ICUs of no CLABSIs for extended periods of time. One simple yet effective means of preventing CRBSI is becoming familiar with appropriate indications for central venous catheters, placing those catheters only when appropriate indications are present, and removing catheters when those indications are no longer present. Steps should also be taken to prevent contamination at the time of insertion, which are often implemented in the form of a bundle, such as that advocated by the Institute for Healthcare Improvement (IHI) (Box 14.2). Emergently placed central lines (i.e., not placed using these techniques) should be removed within 48 hours. Catheter exchanges over a guide wire are discouraged except in rare cases because of high infection rates. A chlorhexidine (CHD)-impregnated sponge applied at the time of placement can further decrease infection rates.
Arterial lines in adults should be placed in the radial, brachial, or dorsalis pedis artery rather than femoral or axillary artery. A minimum of sterile gloves, mask, cap, and a small fenestrated drape are adequate precautions during insertion, except for femoral or axillary locations for which full barrier precautions are required. Transducers and other components of the system (other than the catheter) should be replaced at 96-hour intervals. Disposable transducers are preferred over reusable transducers for infection prevention. Dextrose containing flush solutions should be avoided. A closed flush system should be used, and strict aseptic technique should be maintained when accessing the system.
Management and Treatment
The catheter should be removed if there are local signs of infection or inflammation. If the patient has hypotension, hypoperfusion, or organ failure, and there is no other obvious source of sepsis, the catheter should also be removed immediately. In some patients (e.g., clinically stable with difficult or dangerous to access blood vessels and without a clearly infected catheter), it may be reasonable to manage the patient without immediate catheter removal. In such cases, it may be reasonable to obtain blood culture through the catheter and a peripheral site and to remove the catheter if the DTP is > 120 minutes or if the patient does not improve. After appropriate cultures have been obtained, empiric antibiotics should be started and should include coverage for methicillin-resistant S. aureus (MRSA) and gram-negative rods (GNRs). The choice of antibiotic coverage for GNRs should be based on local antibiotic susceptibility patterns and prior cultures from the patient (Chapter 18). Antipseudomonal coverage should be initiated in all patients with neutropenia, severe illness, or known colonization with Pseudomonas. Antifungal coverage for Candida species should be considered in patients on parenteral nutrition, prolonged broad-spectrum antibiotics, hematologic malignancy, organ or bone marrow transplant, or multisite Candida colonization.
Ventilator-Associated Pneumonia
Definitions
Ventilator-associated pneumonia (VAP) refers to pneumonia that is newly acquired during endotracheal intubation or with a tracheostomy. VAP is not intended to include pneumonias that are acquired prior to intubation and progress to require mechanical ventilatory support. Generally, VAPs arise at least 48 hours after endotracheal intubation. However, the NHSN surveillance definition of VAP includes any pneumonia arising in a tracheally intubated patient regardless of how soon it arises after intubation and pneumonias that arise within 48 hours after extubation. Ventilator-associated tracheobronchitis (VAT) is defined as a lower respiratory infection that arises during tracheal intubation without radiographic evidence of pneumonia. The NHSN has proposed broader surveillance reporting of adverse events during mechanical ventilation, including ventilator-associated event (VAE), ventilator-associated condition (VAC), infection-related ventilator-associated condition (IVAC), possible pneumonia, and probable pneumonia.
VAP is a subcategory of hospital-acquired pneumonia (HAP), which is a pneumonia that is acquired in the hospital and is diagnosed at least 48 hours after admission. HAP is a subcategory of health care–associated pneumonia (HCAP), which is a pneumonia that develops in a patient with certain types of recent medical care. The importance of distinguishing these categories of pneumonia from community-acquired pneumonia (CAP) (Chapter 65) is that these infections are likely to be associated with pathogens that are more antibiotic resistant. Although the concept of VAP is relatively straightforward, there is no agreement on standards for clinical diagnosis (see “Diagnosis”).

Full access? Get Clinical Tree







