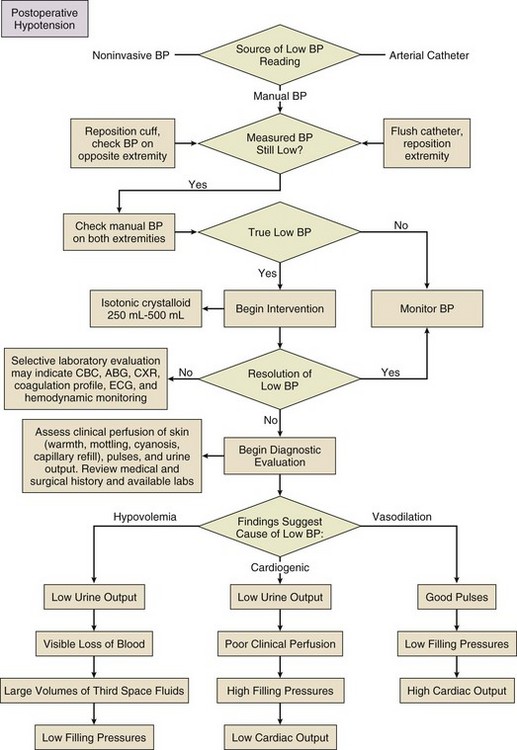
35 Generally, the surgical ICU is where experience, staffing, skills, and technology converge to provide services that cannot be provided anywhere else within the hospital. Highly skilled nurses, often greater in number than the patients themselves, work intimately with intensivists and ancillary staff in an environment designed to stabilize, diagnose, and simultaneously treat the most acutely ill patients. ICU management by intensivists allows for improved staff and family satisfaction, reduced complication rates, lower costs, shorter length of stay, improved processes of care, and a morbidity and mortality risk advantage.1–4 ICU systems focused on an environment of safety and compliance with evidence-based standards promote improvement in many outcome metrics.5 Safe and efficient patient throughput allows for greater institutional procedural volume, which, when paired with surgeon procedural volume, has been shown to be associated with reduced mortality risk.6 Classic postoperative indications for ICU admission include advanced age or prolonged duration of the operation, both criteria without specifically defined thresholds. Other factors, such as the need for mechanical ventilation, volume resuscitation, or administration of vasoactive medications, make ICU care unavoidable. Monitoring of level of consciousness, airway, bleeding, pulses, rhythm, acidosis, urine output, and global perfusion also is facilitated by ICU admission. Identifying patients who may need postoperative ICU care can be difficult. Although there are scoring systems to assess risk and fatality (APACHE, SAPS, MPM, SOFA), it is difficult to apply these predictions to specific disease states or individual patients. Some prediction models utilize physiologic data for patients after admission to the ICU and have not been validated as preadmission screening tools.7,8 Physicians may predict mortality risk even better than scoring systems.9 In practice, most physicians do not use these tools to determine postoperative ICU admission. Admission criteria based on priority, diagnosis, and objective parameter models have been published by the Task Force of the American College of Critical Care Medicine and the Society of Critical Care Medicine.10 Obtaining a comprehensive medical and surgical history is a fundamental step in understanding a patient in the surgical ICU. The medical record, traditionally written but now more commonly electronic, should contain all of the elements necessary to assemble the story up until the time of ICU admission, although deciphering a chart, particularly when it is long, requires time, patience, and detective skills. Data gathering usually begins by word of mouth from the providers delivering the patient. Effective “hand-off” is essential to maintain the continuity of care and to ascertain important operative events that may have escaped documentation. It is in fact a standard expected by The Joint Commission.11 Certain questions are common to virtually all admissions: 2. What are the highlights of the medical/surgical history? 3. Was the operation elective or emergent? 4. What operation was performed, and what are the details of the surgery? 6. What are the current ventilator settings if the patient is intubated? 7. What medications is the patient receiving currently? 8. Where are the vascular access points? Were they placed under sterile conditions? 9. What was the intubation and anesthetic course like? The physical examination of the patient completes the initial postoperative evaluation. It starts as a cursory survey and concludes as a detailed examination. The examination should expose all parts of the patient that can be accessed, and the examiner should inspect and palpate the patient. Areas that are not under examination should be kept covered to preserve body temperature. If the bed sheets are being changed, it presents an opportunity to examine the back of the patient. An initial assessment of the vital signs, skin, pulses, and urine output provides preliminary insight into clinical perfusion (Box 35.1). “Adequate resuscitation” is a state, often temporary, that allows for good clinical perfusion and physiologic stability. Patients with good clinical perfusion (expected heart rates, blood pressures, and urine outputs; absence of acidosis) may require no further resuscitation other than maintenance intravenous fluids. The correct maintenance fluid rate will be just enough to match intravascular losses out of proportion to that which is mobilizable from the interstitium but not so much as to needlessly expand the third space or interstium with edema. Subtle abnormalities in any of these parameters of perfusion may suggest a more serious physiologic derangement warranting further investigation and intervention. Resuscitation is the process of optimizing macroscopic and microscopic metabolic substrate delivery with the goal of avoiding an imbalance between supply and demand. The most fundamental concept is to ensure adequate oxygen delivery (DO2) and meet the oxygen consumption ( Evaluation and optimization of blood pressure, filling pressures, DO2, heart rate, and rhythm often occur simultaneously, particularly in unstable patients (Fig. 35.1). This may require ongoing volume resuscitation and support with vasopressors and inotropes. Restoration of “normal” blood pressure, heart rate, and urine output, however, do not ensure adequate DO2 at the level of the microvasculature.14 Overzealous resuscitation and supranormal DO2 not only do not improve outcome but also may be detrimental.15 Not all patients require the same type of resuscitation. Although the fundamental principles are the same, the particular resuscitation technique end points may differ among the different types of shock.16,17 Crystalloid resuscitation may be appropriate in septic shock but detrimental in the early resuscitation of penetrating traumatic injury.18,19 Even low-volume resucitation plays a role in the management of patients with penetrating traumatic injury or severe intraoperative hemorrhage.20 Early goal-directed therapy with parameter-specific targets has not completely survived prospective validation. However, the principle of timely intervention remains a cornerstone for virtually all types of resuscitation. End points specific to particular mechanisms of injury can vary significantly.21–23 Targeted resuscitation strategies provide an orderly approach to resuscitation, monitoring, and outcome validation. In general, such strategies optimize cardiovascular performance and concurrently measure markers of adequate global DO2 and Resuscitation products should target the intravascular components that are inadequate, including red blood cell concentrates, platelets, coagulation factors, and acellular resuscitation fluids. Fluid type, bolus volume, and maintenance rate must be individualized. The optimal resuscitation fluid effectively should expand the intravascular space and minimize the inflammatory response (particularly in hemorrhagic shock27,28). All resuscitation fluids leak to some degree out of the intravascular space into the interstitium of the extracellular space. Hypotonic resuscitation fluids are inappropriate for volume resuscitation because of their inability to remain exclusively in the extracellular space. Volume per volume, hypertonic fluids cause more intravascular expansion than isotonic fluids. Hypertonic fluids yield no better outcomes than isotonic crystalloids, however, in the resuscitation of trauma patients.29 Similarly, isotonic crystalloids are at least as efficacious or may be better than colloids to reach the same end points.14 In trauma, burn, and general surgery patients, resuscitation with colloids, as compared to crystalloids, has not been shown to reduce the risk of death.30 Metabolic consequences are associated with virtually all resuscitation fluids. Ringer’s lactate can activate neutrophils and cause a potent inflammatory response.31 Hypertonic saline and dextran combinations cause less of an inflammatory response but any mortality benefit is unproved.32,33 Greater than 1 L of hypertonic saline typically results in the development of hypernatremia. Resuscitation exclusively with isotonic NaCl results in a hyperchloremic acidosis. Recent literature has suggested that hetastarch is associated with greater adverse events when compared to saline resuscitation.34 Hetastarch can cause coagulopathy if greater than 1.5 L is given. All acellular resuscitation fluids, if given in sufficient quantities, cause dilutional anemia. As one can infer from this confusing and sometimes contradictory collection of recommendations, no single resuscitation fluid is satisfactory on its own. Postoperative patients can come to the ICU with moderate to severe hypothermia. Heat is lost in the operating room as a result of vasodilation from volatile anesthetics, cool intravenous fluids and air temperature, large open surfaces, and evaporation. Excluding patients with potentially anoxic central nervous system injuries,35 hypothermia complicates initial postoperative care by creating an in vivo coagulopathy, even when in vitro coagulation studies (normalized to 37° C) are normal. In trauma patients, reduction in enzyme activity and platelet function, leading to abnormal fibrin polymerization, occurs at temperatures less than 34° C.36 Care must be taken when administering large volumes of cold blood products or even room temperature crystalloids. Fluid warming devices are available not only to prevent but also to treat hypothermia. All patients with postoperative hypothermia less than 36° C should be actively warmed with forced air blankets, and when normothermia has been achieved, patients should be kept covered to prevent heat loss. Active warming does not cause peripheral vasodilation and subsequent hypotension, and it does not paradoxically cause core cooling owing to heat exchange in cold extremities. Before completing a successful resuscitation, sedation, analgesia, and anxiolysis should be maintained to facilitate patient comfort and to prevent interference with medical care (e.g., mechanical ventilation or motor activity jeopardizing airway, drains, and intravenous catheters). Selected agents should have minimal hemodynamic sequelae and relatively short duration of action so that frequent neurologic assessment can be performed. Daily interruption of continuous sedation has been shown to reduce ICU length of stay, duration of mechanical ventilation, and incidence of posttraumatic stress disorder.37,38 Narcotics such as fentanyl, morphine, and hydromorphone make ideal first-line analgesics. Delivered by continuous infusion and supplemented as needed, successful analgesia reduces pain-driven tachycardia and hypertension and facilitates cough and deep breathing. The sensation of anxiety is a potent dysphoric stimulus that can result in restlessness and interfere with care. Anxiety can be treated with short-acting intravenous benzodiazepines, such as lorazepam. Very short-acting benzodiazepines, such as midazolam, are less useful because of the dosing frequency necessary to prevent symptoms from returning. It is important not to use scheduled benzodiazepines to treat restlessness due to delirium. This practice can exacerbate delirium and worsen outcomes. Delirium can be identified using simple evaluation tools such as the Confusion Assessment Method for the ICU (CAM-ICU). Competitive restlessness due to delirium is best managed with atypical antipsychotics such as haloperidol, ziprazadone, and quetiepine.39 Persistant restlessness, agitation, or delirium can compete with mechanical ventilation, confound hemodynamic stability, and impede the provision of care. If further reduction of level of consciousness is necessary, propofol or dexmedetomidine can be added and titrated to desired effect. Dexmedetomidine, a weak analgesic, can reduce narcotic requirements.40 Propofol, however, has no intrinsic analgesic properties. In a patient who has serious pain, neither propofol nor dexmedetomidine should be used without the concurrent administration of a narcotic. The use of most agents mentioned can be limited by their tendency to reduce blood pressure and, in the case of dexmedetomidine, decrease heart rate. Reentry into consciousness may be accompanied by disorientation, anxiety, pain, and varying degrees of restlessness. In the absence of underlying encephalopathy, it is usually possible to get patients to follow commands, answer questions, and participate in the extubation process. The discomfort of an endotracheal tube can lead to unplanned self-extubation. It is important for the bedside care provider to maintain control of the recovery process by ensuring analgesia and anxiolysis. Small doses of narcotic or benzodiazepine or both can usually correct these problems without inducing further sedation and delay of extubation.41 Patients with encephalopathy resulting from sepsis or shock may not recover a level of consciousness that allows participation in the weaning process. It is controversial whether such a patient should be extubated (avoiding the complications of prolonged extubation) or remain intubated until the ability to protect the airway is more certain. Dexmedetomidine can reduce restlessness without respiratory suppression and may be useful to facilitate extubation of a restless patient. Patients who require sedation for an extended time should receive doses of medication no higher than necessary to achieve the therapeutic target. Sedation scales, such as the Ramsay and Richmond Agitation Sedation Scale,42 are useful to avoid oversedation and ultimately promote earlier liberation from mechanical ventilation. Liberation from mechanical ventilation requires clinical readiness to begin weaning and demonstration of adequate physiologic reserve before extubation. Clinical readiness assesses completion of perioperative tasks at hand and questions any need for early return to the operating room. Resuscitation should be complete, hemostasis should be achieved, metabolic acidosis should be resolving, vasoactive support and gas exchange abnormalities should be minimized, anesthetic agents should be cleared, the ability to protect the airway should be present, and the patient should be awake and reasonably cooperative. These criteria have not been validated clinically, but similar consensus guidelines have been published.43 Daily, if not more frequent, reassessment of clinical readiness is necessary to determine if it is reasonable to consider weaning.44 Patients who do not achieve these basic criteria may require continued mechanical ventilation that maximizes patient comfort and unloads the respiratory muscles. These patients require a structured, evidence-based approach to ventilator weaning and assessment of adequate physiologic reserve. For more detailed information on weaning, refer to Chapter 43. All postoperative ICU patients should be considered for venous thromboembolism (VTE) or deep venous thrombosis (DVT) prophylaxis. The risk of postoperative VTE depends upon both the type of procedure and modifying attributes such as age, prior VTE, history of cancer, obesity, or hypercoagulable state. Risk has been quantified and grouped based on the Modified Caprini Risk Assessment Model.45 Low-risk general and abdominal-pelvic surgery patients should receive intermittent pneumatic compression (IPC) over no prophylaxis or anticoagulant-based prophylaxis. Moderate-risk general and abdominal-pelvic surgery patients should receive anticoagulant-based prophylaxis. Low-dose unfractionated heparin, low-molecular-weight heparin, or fondaparinux should be started in the absence of postoperative bleeding. High-risk general and abdominal-pelvic surgery patients should receive low-dose unfractionated heparin three times a day, low-molecular-weight heparin, or fondaparinux. The highest risk patients should receive mechanical prophylaxis via IPC devices, in addition to low-dose unfractionated heparin, low-molecular-weight heparin, or fondaparinux. In general surgery patients with a high risk of postoperative bleeding, mechanical prophylaxis should be the initial preventive modality until the risk of bleeding has decreased enough to allow for anticoagulant prophylaxis.46
General Principles of Postoperative Intensive Care Unit Care
Postoperative Evaluation
Recovery From Anesthesia
Postoperative Resuscitation
Assessment
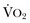 ) needs of tissues and organelles. Because the moment when
) needs of tissues and organelles. Because the moment when 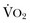 exceeds DO2 is difficult to determine, resuscitation “targets” serve as proxy markers of adequate DO2. Resuscitation targets are reproducible, quantifiable values, such as pressures, outputs, metabolites, inflammatory mediators, or oxygen saturations, which represent therapeutic goals. Resuscitation targets provide an important opportunity for study and outcome validation. Despite the seemingly simple logic of employing resuscitation targets, few of these therapeutic goals have been shown to improve clinical outcome. Even routine data derived from a pulmonary artery catheter have not been shown to improve outcome in patients undergoing surgery with decompensated cardiogenic shock or acute lung injury.12,13
exceeds DO2 is difficult to determine, resuscitation “targets” serve as proxy markers of adequate DO2. Resuscitation targets are reproducible, quantifiable values, such as pressures, outputs, metabolites, inflammatory mediators, or oxygen saturations, which represent therapeutic goals. Resuscitation targets provide an important opportunity for study and outcome validation. Despite the seemingly simple logic of employing resuscitation targets, few of these therapeutic goals have been shown to improve clinical outcome. Even routine data derived from a pulmonary artery catheter have not been shown to improve outcome in patients undergoing surgery with decompensated cardiogenic shock or acute lung injury.12,13
Management Theory
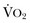 . Increased serum lactate concentration, decreased mixed venous oxygen saturation, and decreased central venous oxygen saturation are the proxy markers for inadequate global DO2. However, normal values of mixed venous oxygen saturation and central venous oxygen saturation do not guarantee normal use of oxygen in the tissues, particularly at the regional level. Appropriate targets for microcirculatory resuscitation remain elusive. Noninvasive techniques have reduced the need to obtain physiologic data by the use of a pulmonary artery catheter.24 Pulse and pressure wave analysis along with their derivitives (cardiac output and stroke volume variation) offer a less invasive way of measuring hemodynamic performance and predict volume responsiveness in the appropriate patient population.25 Gastric tonometry, sublingual capnography, near-infrared spectroscopy, and orthogonal polarization spectral imaging are less mainstream technologies available to assess the effectiveness of resuscitation at the regional level.26
. Increased serum lactate concentration, decreased mixed venous oxygen saturation, and decreased central venous oxygen saturation are the proxy markers for inadequate global DO2. However, normal values of mixed venous oxygen saturation and central venous oxygen saturation do not guarantee normal use of oxygen in the tissues, particularly at the regional level. Appropriate targets for microcirculatory resuscitation remain elusive. Noninvasive techniques have reduced the need to obtain physiologic data by the use of a pulmonary artery catheter.24 Pulse and pressure wave analysis along with their derivitives (cardiac output and stroke volume variation) offer a less invasive way of measuring hemodynamic performance and predict volume responsiveness in the appropriate patient population.25 Gastric tonometry, sublingual capnography, near-infrared spectroscopy, and orthogonal polarization spectral imaging are less mainstream technologies available to assess the effectiveness of resuscitation at the regional level.26
Temperature Control
Awakening from Anesthesia
Postoperative Extubation
Best Practices
Prevention of Venous Thromboembolism and Deep Venous Thrombosis

Full access? Get Clinical Tree


General Principles of Postoperative Intensive Care Unit Care