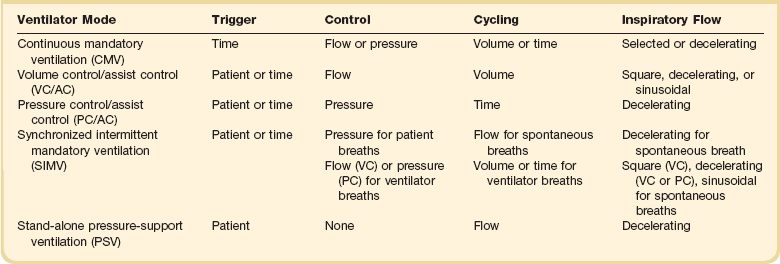
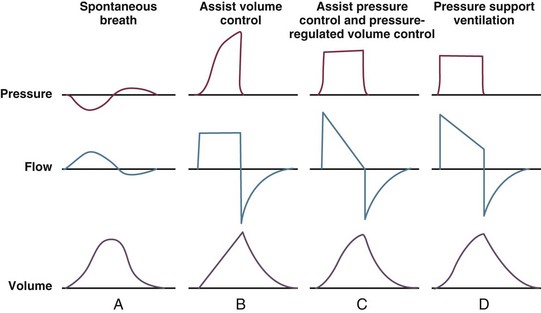
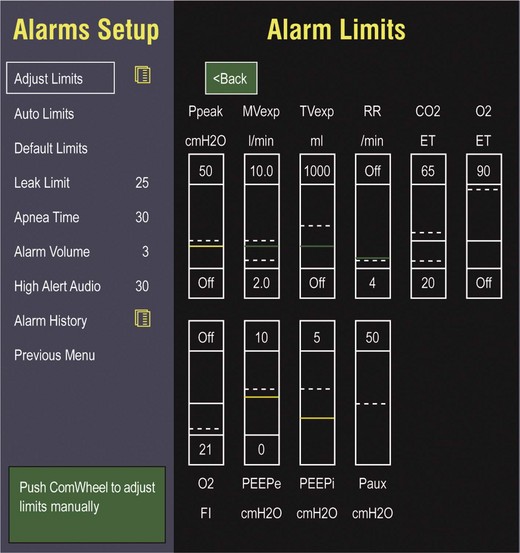
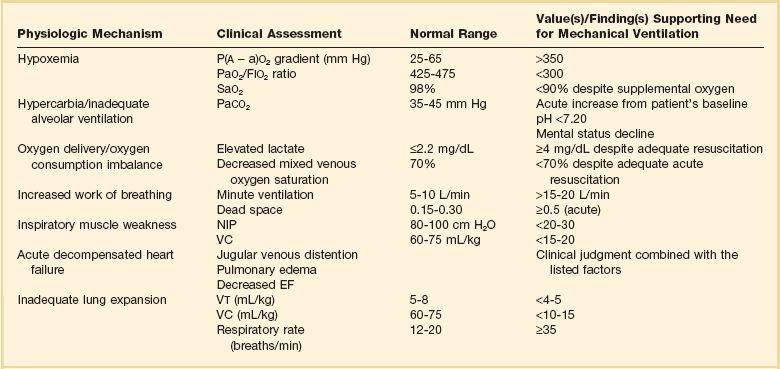
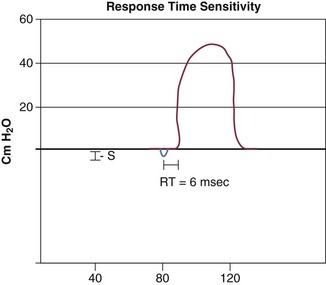
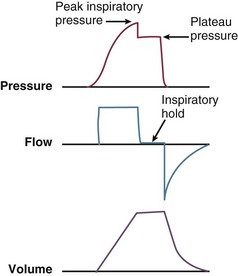
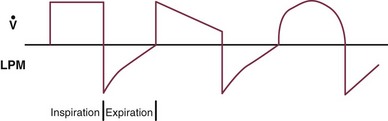
9 INDICATIONS FOR MECHANICAL VENTILATION Continuous Mandatory Ventilation Synchronized Intermittent Mandatory Ventilation Stand-Alone Pressure Support Ventilation OTHER MODES OF MECHANICAL VENTILATION POSITIVE END-EXPIRATORY PRESSURE MONITORING THE VENTILATED PATIENT MAINTAINING SUPPORT OF THE VENTILATED PATIENT WEANING FROM MECHANICAL VENTILATION COMPLICATIONS OF MECHANICAL VENTILATION Management of the mechanically ventilated patient is a cornerstone of critical care training and practice. The institution of mechanical ventilation can be a lifesaving measure. However, the mechanical ventilator also has potential for great harm and, in and of itself, does not reverse underlying disease. Limiting iatrogenic injury from ventilator-induced lung injury (VILI) should take high priority, along with acceptable levels of oxygenation and ventilation. The clinician should be aware of basic and advanced principles involving mechanical ventilation, allowing flexibility when applying evidence-based practices to the individual patient. Knowledge of guidelines and large clinical trials is vitally important, and the consideration of patient trajectory, individual physiology, timing of therapy, and severity of illness will make tailoring the ventilator prescription most effective.1 Hippocrates (460-370 BC) likely gave the first description of endotracheal intubation in his “Treatise on Air,” in which he states that “One should introduce a cannula into the trachea along the jawbone so that air can be drawn into the lungs.”2 In 1530, Paracelsus (1493-1541) used a fire bellows connected to a tube inserted into a patient’s mouth as a ventilator device.3 The first known mechanical device designed specifically to provide ventilation for the patient was the foot pump developed by Fell and O’Dwyer in the 1880s.4 The first generation of mechanical ventilators focused primarily on the intermittent delivery of a bulk volume of gas to the patient with limited monitoring.5 Negative-pressure ventilators were invented and applied a negative pressure around the body or chest cavity. Two classic devices that provided negative-pressure ventilation were the iron lung and the chest cuirass or chest shell.6 Iron lungs were widely used during the poliomyelitis epidemics of the 1930s and 1940s. These devices encased the patient from the neck down and applied negative pressure around the patient to expand the lungs. The chest cuirass was intended to alleviate the problems of patient access and “tank shock” that occurred secondary to venous pooling during the application of negative pressure associated with iron lungs.7 Although the chest cuirass improved patient access and decreased the potential for tank shock, ventilation with this device was limited by the difficulties in maintaining an airtight seal between the shell and the patient’s chest wall. After the polio epidemic of the 1960s, the era of respiratory intensive care emerged, as positive-pressure ventilation via an artificial airway became commonplace.6 Controlled mechanical ventilation eventually led to assisted modes of support, and positive end-expiratory pressure (PEEP) was introduced in the late 1960s. The improvements in mechanical ventilators came about as understanding was gained in manipulating variables of flow and pressure for patient benefit. Further technical evolution of ventilators included advances such as intermittent mandatory ventilation, and synchronous intermittent mandatory ventilation. Modern ventilators now boast microprocessors that serve both in the operating mechanism of the device and in the monitoring systems, enabling automatic adjustment of most aspects of the mechanical breath being delivered. Although modern ventilators have evolved into complex machines, the basic premise remains: a ventilator is designed to replace or augment a patient’s muscles in performing the work of breathing.8 Ventilators use input power (electricity or compressed gas) to ventilate the lungs. To generate a breath (whether it be spontaneous or positive pressure), a pressure gradient must be generated from the airway opening to the alveoli. The volumes delivered and pressures generated largely depend on the mechanical properties of the respiratory system: the lungs and chest wall as well as the abdomen.8 Each of these components has mechanical properties that determine the overall behavior of the respiratory system. Although the respiratory system can be quite complex, the main variables of interest are pressure, flow, and volume. The ventilator must generate a pressure to cause flow through an open circuit and therefore increase lung volume.9 The pressure required to do this reflects a combination of the pressures to inflate the lung and chest wall. This can be illustrated by the equation of motion:9,10 Compliance describes the ease or difficulty of the respiratory system to expand in response to a delivered pressure and volume. Simplistically, compliance is defined by the change in volume (ΔV) divided by the change in pressure (ΔP), and the compliance of the respiratory system (CRS) is ΔV/ΔPalveolar. Elastance is the inverse of compliance, or the ratio of pressure change to volume change, and describes the tendency to recoil. Resistance describes the impedance to airflow through the respiratory system, or the ratio of pressure change to flow change. The elastic load is the pressure required to overcome the elastance of the respiratory system, and the resistive load is the pressure required to overcome flow resistance of the ventilator circuit, endotracheal tube, and airways.8 The ventilator circuit consists of plastic tubing connecting the artificial airway or mask with the mechanical ventilator. Within the circuit may reside humidifiers, devices for the delivery of aerosolized medications, filters, suction catheters for secretion clearance, and heated wires.11 The length and compliance (2-3 cm3/cm H2O) of the ventilator circuit are responsible for a volume of gas contained within the circuit, termed the compressible volume. This is partly responsible for the discrepancy between the set tidal volume delivered and the expiratory volume measured and displayed by the ventilator. The ventilator circuit also adds resistance to the system, but is minimal when compared to either the patient’s inherent mechanics or the endotracheal tube. Although frequently colonized with bacterial pathogens, the routine change of the ventilator circuit for infection prevention (e.g., ventilator-associated pneumonia) is not recommended. An HME, which is placed between the artificial airway and the ventilator circuit, may be used to replace the traditional heated humidifier. During exhalation, moisture and heat from the patient are absorbed into the honeycomb structure of the exchanger and are transferred back to the patient during the next inhalation. Ventilator circuits with bacterial-viral filtering HMEs cost less to maintain and are less likely to colonize bacteria than those with heated humidifiers.12,13 Contraindications for use of an HME are thick or large amounts of secretions, minute volume exceeding 10 L/minute, body temperature less than 32° C, and need for aerosolized medications.14 Current ventilators are equipped with monitors that constantly or periodically assess the ventilator’s operation and the patient’s status (Fig. 9.1). These monitors are usually associated with alarms that visually or audibly notify the operator of any variation from the preset norm. Ventilator alarms can warn of potentially life-threatening events, must have an appropriate level of sensitivity and specificity, and must be evaluated clinically and in a clinical context.15 There are some alarms related to power input (e.g., low battery, loss of power, loss of air supply) and control circuit (e.g., nonfunctioning ventilator, incompatible settings), but most alarms are related to measured output, such as pressure, volume, and flow.8 High-pressure alarms are triggered by patient factors (such as decreased compliance and increased resistance of the respiratory system) or by ventilator circuit malfunction (obstruction or kinking of the endotracheal tube). Low-pressure alarms are generally secondary to a leak in the system (ventilator circuit, endotracheal tube or cuff) or patient (large pressure loss from a bronchopleural fistula). A high expired volume alarm could be seen with improved pulmonary mechanics during pressure-control ventilation. Low expired volume could be secondary to patient-ventilator disconnect or a leak in the system or patient. A high-frequency alarm is secondary to either autotriggering or hyperventilation, and a low-frequency alarm indicates bradypnea or apnea.8,15 Traditionally, most clinicians apply some amount of pressure support during inspiration to compensate for the increased work of breathing related to artificial airway resistance. The amount of pressure support needed to counterbalance this resistance is highly variable, depending not only on the internal diameter of the endotracheal tube but also on flow, bend of the tube, and changing demands of the patient. This variability makes one level of pressure insufficient to meet these changing demands.16 Automatic tube compensation (ATC) compensates for endotracheal tube resistance via closed-loop control of calculated tracheal pressure. A ventilator with ATC compensates for the pressure drop across the endotracheal tube during inspiration by increasing the airway pressure and during expiration by decreasing airway pressure according to actual gas flow.17–19 This technique uses a continuous calculation of the flow-dependent drop in pressure across the endotracheal tube. ATC is similar to PSV, but the pressure applied by the ventilator varies as a function of endotracheal tube resistance and flow demand. Most of the interest in ATC revolves around eliminating the imposed work of breathing during inspiration. During expiration, however, ATC may also compensate for that flow resistance by lowering the pressure in the expiratory limb transiently from its PEEP setting, helping reduce effective expiratory resistance and auto-PEEP.17,20 In addition to overcoming the work of breathing imposed by the artificial airway, ATC may improve patient-ventilator synchrony by varying the flow commensurate with demand and may reduce air trapping by compensating for imposed expiratory resistance. During weaning trials, this technique may allow a more reliable prediction of patient performance when the tube is removed. Mechanical ventilation is instituted for a number of reasons (Table 9.1).21 Most commonly, these indications are a combination of a failure to adequately oxygenate, ventilate, or meet the metabolic demands of a physiologically stressed patient. Clinical indicators such as tachycardia, arrhythmias, hypertension, and tachypnea, use of accessory respiratory muscles, diaphoresis, and cyanosis are used to diagnose respiratory distress. Type I respiratory failure is hypoxemic respiratory failure, defined as a partial pressure of oxygen in arterial blood (PaO2) less than 60 mm Hg. Type II respiratory failure is hypercarbic respiratory failure, defined as PaCO2 greater than 50 mm Hg, if elevated from patient baseline and associated with acidosis.22 Blood pH is generally a better indicator than PaCO2 for adjusting minute ventilation. Hypercapnia should not prompt aggressive intervention if pH remains acceptable and the patient remains alert. Hypercapnia is generally well tolerated, but this clearly depends on the underlying pathophysiology and comorbid conditions of the patient (e.g., right ventricular dysfunction). However, a sustained pH of 7.65 or greater or 7.10 or less is often considered sufficiently dangerous in itself to require control of minute ventilation by mechanical ventilation. Mechanical ventilation may also be instituted to maintain normal blood pH, decrease work of breathing, assist left ventricular function in the setting of acute decompensated heart failure, or for airway protection in the setting of toxic overdose, traumatic brain injury, or any other significant acute central nervous system illnesses. Box 9.1 suggests guidelines for setting basic operating parameters in a mechanical ventilator. Triggering represents the change from expiration to inspiration and occurs either because of a drop in circuit pressure or diversion of flow (when patient triggered), or because of elapsed time. Sensitivity refers to a preset threshold of pressure or flow. When this threshold is reached, a mechanical breath is delivered. This threshold can be adjusted, and is usually set at −1 to −2 cm H2O. If sensitivity is set too low, the ventilator will be triggered by any process that causes the airway pressure to drop below the set threshold. Such processes include patient motion, external compression, gastric suctioning, and air leaks in the circuit. Conversely, if the threshold is set too high, the work of breathing increases, as the patient must make a significant effort to overcome the threshold limit for inspiratory flow to occur. In the setting of pressure triggering, airway pressure is reduced (by patient effort) in the proximal circuit, the expiratory valve closes, pressurization of the inspiratory limb of the circuit occurs, and the patient receives a breath. Flow sensing was developed as an alternative to pressure triggering to reduce the delay in response time between neural input from the patient and delivery of gas volume by the ventilator.23,24 In the setting of flow triggering, the patient’s inspiratory effort induces a disruption of the constant flow in the ventilator inspiratory circuit. This change in flow signals the expiratory valve to close and for the ventilator to deliver the next breath. Flow triggering was initially demonstrated to decrease the work of breathing when compared to pressure triggering. However, with improvement in response time, monitoring, and feedback, pressure and flow triggering are similar in this regard.25,26 Figure 9.2 is a representation of patient effort and ventilator response time. During inspiration, pressurized gas is channeled from the ventilator to the patient after the exhalation valve closes. This phase can be controlled by how one sets flow or pressure in the ventilator proximal to the open inspiratory valve. For example, volume-assist control is flow controlled and pressure-assist control is pressure controlled. Choice of control variable is largely the discretion of the clinician, as either can be manipulated to achieve set goals. It should be noted, though, that in volume-targeted ventilation, excessive airway pressures can arise secondary to worsening pulmonary mechanics. In this situation, the pressure alarm will cause a pressure limit to cycle to expiration. At the end of inspiration in volume-targeted ventilation, an inspiratory hold maneuver can be performed (Fig. 9.3), which can distinguish the peak airway pressure from the plateau pressure (because flow is stopped, resistance is negligible). In pressure-targeted ventilation, minute ventilation is not guaranteed. It is a function of the compliance and resistance of the respiratory system. The clinician therefore should monitor these physiologic changes closely to avoid untoward changes in either airway pressure or PaCO2 levels. With pressure control and pressure support (PS) breaths, the pattern of inspiratory flow is a natural decelerating pattern as the pressure gradient for flow decreases as pressure rises in the patient’s lungs. The pattern of inspiratory flow with a flow-controlled breath is naturally square but can be computer altered to be decelerating or sinusoidal (Fig. 9.4). A square inspiratory flow pattern results in a rapid rise to a preset level (set by the clinician) followed by constant flow until cycling occurs. Decelerating flow results in a rapid rise to a maximum level followed by a gradual decrease until cycling. Sinusoidal flow pattern most closely represents normal physiologic breathing. It results in flow that gradually increases and then decreases during inspiration. The choice of inspiratory flow pattern should be based on patient characteristics, and a few common clinical scenarios should be familiar to the clinician. Square flow over time results in a shorter inspiratory time (I time), and therefore longer expiratory time (E time). For this reason it may be preferred in patients with obstructive physiology (chronic obstructive pulmonary disease [COPD] or asthma).27,28 It is also usually tolerated better in patients with demand for high minute ventilation, such as severe metabolic acidosis or elevated ICP. In this situation, there is a potential for dyssynchrony to occur during the progression of the inspiratory phase if decelerating flow is chosen. The tradeoff is higher peak airway pressures with a square waveform, which may be ameliorated if it leads to a reduction in hyperinflation and iPEEP. Decelerating flow results in a longer I time and likely a better distribution of flow. With pulmonary pathophysiology involving heterogeneous distribution of injury (ALI as the prototype example), decelerating flow is likely the best choice, as the longer I time can lead to more homogeneous distribution of ventilation. The distribution of ventilation can be quite different when the flow pattern changes. A decelerating flow pattern yields the most even distribution under most abnormal airway conditions.29 Studies have also demonstrated that a decelerating flow pattern improves the geographic distribution of lung vibration as a presumed surrogate of airflow.30 The length of inspiration depends on several factors. In the pressure control mode of ventilation, the clinician directly controls the I time and I : E ratio. With pressure support the patient primarily controls I time. In flow-controlled (volume-targeted) ventilation, the I time is a function of set tidal volume (VT) and flow rate (VT/inspiratory flow rate) as well as flow wave characteristics (shape). The end-inspiratory alveolar pressure, as measured by an end-inspiratory hold, is the same for a given tidal volume regardless of the type of ventilator breath. A ventilator mode describes ventilation over time and a set of specific combinations of breath characteristics delivered to a patient, including types of breaths, phase variables, and mandatory versus spontaneous breaths.16 Clinician familiarity, unit, and institutional practice patterns determine to a large extent the mode that is employed. Also, data showing definitive improvement in clinically relevant outcomes when comparing one mode to another are lacking. Patient need and response to therapy should guide mode selection. Table 9.2 summarizes the features of some of the modes of mechanical ventilation, and Table 9.3 lists some of their advantages and disadvantages. Figure 9.5 also shows some characteristic waveforms of various modes of mechanical ventilation. Table 9.3 Potential Advantages and Disadvantages of Selected Modes of Mechanical Ventilation
General Principles of Mechanical Ventilation
History
Mechanical Ventilation
Basic Concepts
The Ventilator Circuit
Alarms and Safety
Automatic Tube Compensation
Indications for Mechanical Ventilation
Mechanical Breath Generation
Ventilator Modes
Mode
Advantage(s)
Disadvantage(s)
Controlled mechanical ventilation (CMV)
Rests muscles of respiration
Requires use of heavy sedation/neuromuscular blockade
Assist volume control (AVC)
Reduced work of breathing
Guarantees delivery of set tidal volume (unless peak pressure limit alarm is exceeded)
Potential adverse hemodynamic effects
May lead to inappropriate hyperventilation and excessive inspiration pressures
Assist pressure control (APC)
Allows limitation of peak inspiratory pressures
Same as for AVC
Potential hyperventilation or hypoventilation with lung resistance/compliance changes
Synchronized intermittent mandatory ventilation (SIMV)
Less interference with normal cardiovascular function
Increased work of breathing compared with assist control
Patient may find it difficult to adjust to two different types of ventilator breaths
Stand-alone pressure-support ventilation (PSV)
Patient comfort
Improved patient-ventilator interaction
Decreased work of breathing
Apnea alarm is only backup
Variable patient tolerance
General Principles of Mechanical Ventilation

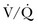 ) mismatch occurs when areas of the lung are perfused but either poorly ventilated (low
) mismatch occurs when areas of the lung are perfused but either poorly ventilated (low 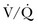 ) or not ventilated at all (shunt). The latter is an intrapulmonary (IP) (capillary) shunt. Shunt may also be intracardiac (anatomic). Venous admixture, as a measure of less than fully oxygenated blood after passing through the lung, includes both low
) or not ventilated at all (shunt). The latter is an intrapulmonary (IP) (capillary) shunt. Shunt may also be intracardiac (anatomic). Venous admixture, as a measure of less than fully oxygenated blood after passing through the lung, includes both low 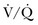 areas of lung and IP shunt. Normal venous admixture is about 2% to 5%. Mechanical ventilation may increase the venous admixture to approximately 10% in the normal individual. Mechanical ventilation usually decreases venous admixture in alveolar lung disease, such as acute lung injury (ALI), improving the distribution of ventilation especially in previously underventilated lung areas. Pressures greater than alveolar opening and closing pressures expand the collapsed alveolus and prevent its collapse, respectively. However, if positive-pressure ventilation produces overdistention, redistribution of pulmonary blood flow to unventilated regions may occur, resulting in hypoxemia. Dead space refers to areas of the lung with a higher
areas of lung and IP shunt. Normal venous admixture is about 2% to 5%. Mechanical ventilation may increase the venous admixture to approximately 10% in the normal individual. Mechanical ventilation usually decreases venous admixture in alveolar lung disease, such as acute lung injury (ALI), improving the distribution of ventilation especially in previously underventilated lung areas. Pressures greater than alveolar opening and closing pressures expand the collapsed alveolus and prevent its collapse, respectively. However, if positive-pressure ventilation produces overdistention, redistribution of pulmonary blood flow to unventilated regions may occur, resulting in hypoxemia. Dead space refers to areas of the lung with a higher 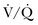 ratio. Anatomic dead space is the volume of the conducting airways of the lungs, about 150 mL. Alveolar dead space refers to alveoli that are overventilated relative to perfusion; it is increased by any condition that reduces pulmonary blood flow, such as pulmonary embolism (PE) or with overdistention of the lung. Mechanical dead space refers to the rebreathed volume of the ventilator circuit; this volume behaves like an extension of the anatomic dead space. Mechanical ventilation can also increase dead space if it leads to overdistention.
ratio. Anatomic dead space is the volume of the conducting airways of the lungs, about 150 mL. Alveolar dead space refers to alveoli that are overventilated relative to perfusion; it is increased by any condition that reduces pulmonary blood flow, such as pulmonary embolism (PE) or with overdistention of the lung. Mechanical dead space refers to the rebreathed volume of the ventilator circuit; this volume behaves like an extension of the anatomic dead space. Mechanical ventilation can also increase dead space if it leads to overdistention.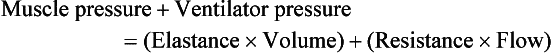
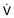 , flow.
, flow.