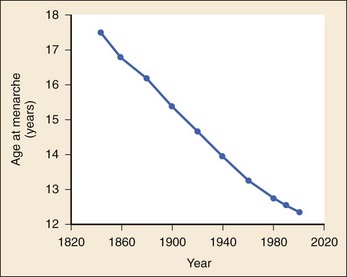
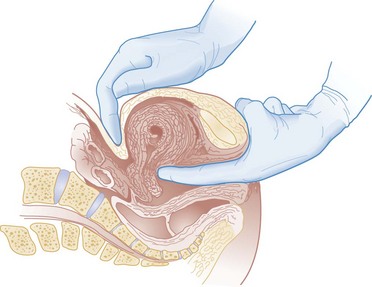
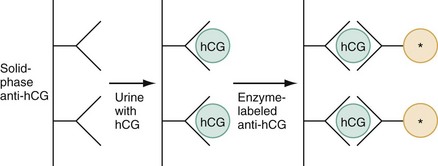
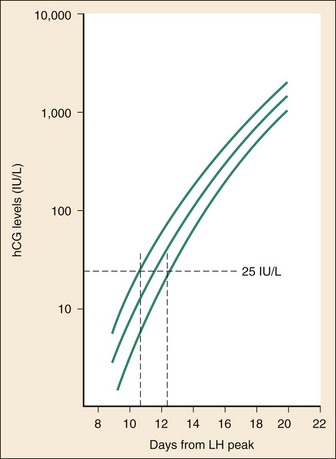
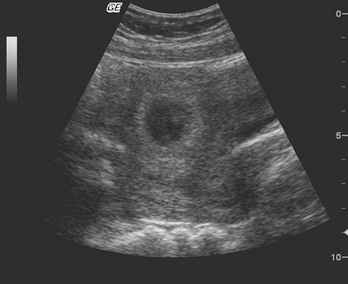
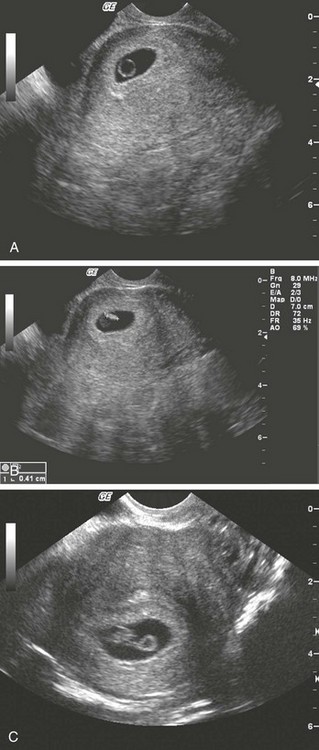
Chapter 177 The 2008 report from the U.S. National Center for Health Statistics noted that the birth rate for women younger than 25 years, the principal childbearing age, had fallen from 43% in 1990 to 34.4% in 2009.1 The overall birth rate for teens aged 15 to 19 years dropped from 15% in 1990 to 10% in 2009,1 which resulted in a historic low of 66.7 pregnancies per 1000 women.2 Nearly half of all pregnancies in the United States occur among unmarried women, and this number is rising, increasing from 2.7 million in 1990 to 2.8 million in 2004, whereas rates among married women continue to fall, from 4.1 million in 1990 to 3.5 million in 2004 (newer data do not exist at the time of publication). Pregnancy outcomes in the United States vary across the black, Hispanic, and white populations. The rate of pregnancies resulting in live births is highest in Hispanic women (98%) compared with non-Hispanic whites (69%) and non-Hispanic blacks (50%), whereas the rate of pregnancy resulting in abortion is highest in non-Hispanic blacks (37%) compared with non-Hispanic whites (12%) and Hispanic women (19%).1 The median age at menarche for adolescent American girls fell from 15.5 years in 1900 to 12.3 years in 20003 (Fig. 177-1). From 1980 to 2008, the number of twin births in the United States rose more than 100% (from 68,339 to 138,660), and triplet and higher order births rose just less than 400% (from 1377 to 6268 births).2 At the same time, differences in twin birth rates between non-Hispanic white and non-Hispanic black mothers disappeared. Non-Hispanic white women were more than twice as likely as non-Hispanic black or Hispanic women to have a triplet birth.2 Maternal mortality related to complications of pregnancy and childbirth in the United States has slowly been declining during the past several years from 2960 maternal deaths in 1950 to 548 deaths in 2007.4 The highest number of deaths is attributed to women older than 35 years, with 32 deaths per 100,000 live births, which is four times the rate for women 29 years of age and younger.2 The most common causes of death are pulmonary embolism, diseases of the circulatory system, and postpartum hemorrhage.5 Rates of elective cesarean deliveries in industrialized countries are continuing to rise secondary to a common perception of safety and potential benefits of elective low-risk cesarean delivery compared with vaginal delivery. In Canada, the rate of planned cesarean deliveries has increased to 26% in 2003 compared with 5% in 1969.4 A retrospective cohort study, which evaluated more than 2.3 million healthy pregnant patients, demonstrated a composite severe morbidity of 27 per 1000 deliveries for planned cesarean delivery for breech presentation versus 9 per 1000 deliveries for planned vaginal delivery for any reason, respectively.4 Furthermore, although the study showed an increased risk of maternal cardiac arrest of 2% in the planned cesarean delivery group and 0.4% in the vaginal delivery group, the difference in the rate of in-hospital maternal death was nonsignificant (P = .87). Decisions about planned cesarean sections for other than conventional indications require a discussion of the risks and benefits of this intervention. Statistical data concerning pregnant patient demographics and frequency of use of the ED and the diagnostic profile are incomplete because most observational data sets group perinatal and pregnancy as “other medical diagnosis,” which accounts for less than 2% of visits to the ED in the United States and Canada5; however, persons between the ages of 18 and 44 years account for approximately 22% of all ED visits, so the risk of an unknown pregnancy complicating the diagnosis may be higher than anticipated.6 A study of random pregnancy tests performed on female trauma patients revealed that 2% were pregnant.7,8 Even when the chance of pregnancy was remote, female patients seeking treatment in the ED had a pregnancy rate of at least 10%.7 Maternal cardiac arrest is rare, although recent data from the Confidential Enquiries into Maternal and Child Health data set show a maternal cardiac arrest frequency that is on the rise with a current rate of 1 : 20,000 pregnancies, up from the previous rate of 1 : 30,000.9 Pregnant patients may present with symptoms and signs related to an undiagnosed, normal pregnancy (abdominal discomfort, nausea, urinary frequency, breast swelling and tenderness, fatigue, or near-syncope), symptoms and signs related to complications of pregnancy (see Chapter 178), or clinical manifestations of chronic medical illnesses that may be exacerbated by pregnancy (see Chapter 179). This chapter covers the following aspects of a normal pregnancy: common initial signs and symptoms, diagnostic tests, and considerations for treatment and referral. The reliability of the sexual and menstrual history has been notably poor. Ramoska and colleagues reported that 11.5% of women with abdominal pain or vaginal bleeding had positive pregnancy test results, in contrast to their perception that there was “no chance” they were pregnant.7 Missed diagnosis of pregnancy in adolescents was associated with failure to document a sexual or menstrual history. Adolescents rarely mentioned the possibility of pregnancy at triage (10% of those found to be pregnant), and 10% of those with a positive pregnancy test result denied being sexually active.10 Interestingly, pregnancy rates in adolescents continue to rise in the United States, with estimates of 42.5 per 1000 women of this age group, and have increased 5% from 2005-2007 data.1 Thus one should have a high index of suspicion of pregnancy in adolescents who present with symptoms of pregnancy, which include cessation of menses, anorexia, nausea, easy fatigability, urinary frequency, breast swelling and tenderness, and perception of fetal movement or “quickening” (beginning at 16 to 20 weeks). Cessation of menses becomes suggestive of pregnancy at 10 days or longer after the time that menstruation is expected. Intermittent, spontaneous vaginal bleeding or vaginal discharge is common during the first half of pregnancy, particularly in multiparous women. Most women without cervical lesions experience bleeding on or before the 40th day of pregnancy. This bleeding has been interpreted by some investigators to be physiologic in origin and a result of implantation.11 Nevertheless, any patient presenting with vaginal bleeding during pregnancy should be considered to be at risk for a pathologic cause of the bleeding. It is possible to calculate the estimated date of delivery in a regularly menstruating woman who has 28-day cycles and can give a reliable history. Franz K. Nägele, a German obstetrician in the 18th century, developed Nägele’s rule, which is still the current standard: add 7 days to the date of the first day of the last menstrual period and subtract 3 months. Currently, with the increasing use of smart phones, hand-held devices, and the Internet, literally hundreds of calculators are available to clinicians to aid in the estimation of delivery date. In the absence of reliable recall of the first day of the last menstrual period, first-trimester ultrasonography may estimate the date of delivery. Ultrasonography dating is most accurate if it is done between 10 and 12 weeks.12 Vital signs in a pregnant patient are essentially the same as those in a nonpregnant patient. The only changes are seen in the heart rate and blood pressure. The resting heart rate increases approximately 10 to 15 beats/minute, and blood pressure demonstrates a mean arterial decrease of 6 to 10 mm Hg, which reaches a nadir in mid–second trimester and slowly increases to the patient’s nonpregnant value by term. Blood pressure in the brachial artery is highest in the sitting position, lowest when lying in the left lateral position, and intermediate in the supine position, except in pregnant women who have supine hypotension syndrome. Pathologic hypertension of pregnancy is defined as a sustained rise of 30 mm Hg systolic or 15 mm Hg diastolic above baseline values on at least two occasions, 6 hours or more apart. A study demonstrated a steady increase in prevalence of hypertension in pregnant women from 67 per 1000 pregnant patients in 1998 to 83 per 1000 patients in 2006. The highest risk cohort is women older than 35 years, with a reported rate of 89 per 1000 patients.13 The authors concluded that hypertension and related illnesses are major risk factors for severe obstetric morbidity. Furthermore, as more women have children later in life, in addition to the increasing prevalence of obesity, the number of patients with hypertension-related illness due to pregnancy will most likely continue to rise.13 Fetal heart activity should be included as the fifth vital sign in the evaluation of a pregnant patient. It can be auscultated with a stethoscope at 17 weeks of gestation on average and by 19 weeks in nearly all pregnancies in nonobese women. With the use of Doppler ultrasonography, fetal cardiac activity can be detected as early as 8 weeks; however, it is more commonly heard by 10 weeks. With improved transvaginal ultrasonography technology, it has been possible to detect fetal cardiac activity in embryos as small as 6 mm.14 Hegar’s sign, which is softening of the lower uterine segment caused by hyperemia, can be appreciated during an internal pelvic examination at approximately 6 to 8 weeks of pregnancy. In some cases the softening is sufficient to allow the examiner to easily distinguish the cervix from the fundus on palpation. It can be found in other conditions, but it is not as easily detected as it is in pregnancy (Fig. 177-2). The vaginal mucosa becomes hyperemic during pregnancy and with any other condition that causes congestion of the pelvic organs. In response, the color of the mucosa of the vaginal walls changes from pink to blue to violet and can be visualized at the vaginal introitus (Chadwick’s sign).15 At midpregnancy the fetus can be detected by ballottement or palpated through the maternal abdominal wall in a nonobese patient. The fetus floats in a large volume of amniotic fluid. Thus, with ballottement, pressure exerted on the uterus causes the fetus to sink and then to rebound to its original position. Palpation of the outline of the fetal body becomes easier near the end of pregnancy; however, subserosal myomas can simulate the fetal head and small parts, or both.10 The fundamental process of ELISA involves an antibody adherent to a solid-faced support (usually plastic) that binds to a region of the hCG molecule. A second antibody chemically linked to an enzyme (e.g., alkaline phosphatase) binds to another side of the trapped hCG molecule. The hCG molecule is sandwiched between the two antibodies. Excess enzyme-linked antibody is washed away, and a color developer is added and becomes blue when it comes in contact with the enzyme (Fig. 177-3). The antibodies can be polyclonal or monoclonal. Many variations exist with respect to the solid-faced support and the method of developing the color reaction. In 1988 the first one-step pregnancy test became available. It was developed and patented by Unipath and sold as Clearblue One Step. This test uses monoclonal antibodies specific for the beta subunit of hCG. It includes a unique built-in control that shows a blue line when the test is complete and performed correctly.16 This test appeals to consumers because it is rapid (requiring less than 5 minutes), requires no manipulation by the user, offers hygienic sample handling, provides clear and easy-to-read results, and has a sensitivity level of hCG between 25 and 50 mIU/mL. This level of sensitivity means that more than 95% of pregnancies within 3 days after missed menses will be detected by a home pregnancy device.17 For detection to occur on the day of missed menses, a lower sensitivity level of 12.5 mIU/mL is required. A study in 2001 compared routine urinary hCG point-of-care qualitative tests performed by nurses in the ED with laboratory tests and found the former to be equally accurate and much faster, shaving 35 minutes off wait times for results.18 A well-designed ELISA applied to urine is not affected by drugs or a concurrent physiologic state. The only exception is patients taking exogenous hCG for induction of ovulation.19 Normal hCG levels in men and premenopausal women range from 0.02 to 0.8 mIU/mL. Postmenopausal women may have higher levels.20 The blastocyst begins to secrete hCG 7 days after fertilization.21 Thus the hCG released can be detected as early as 6 to 8 days after conception.22 The initial doubling time is fast and has been attributed to the actual process of implantation. This is followed by a slower rate of doubling as trophoblast hCG and maternal circulatory hCG levels equilibrate22 (Fig. 177-4). Levels of hCG peak at 7 to 10 weeks of pregnancy, with a mean value of 50,000 mIU/mL and a range of 20,000 to 200,000 mIU/mL.21 The test results for hCG become positive in 98% of patients 7 days after implantation, which coincides with the time of the expected period. When a test with a sensitivity of 25 to 50 mIU/mL is used, a negative result 1 week from the expected time of the missed period essentially guarantees that a woman is not pregnant.19 False-positive results can be attributed to postmenopausal status, abortion in the first trimester, exogenous hCG for induction of ovulation, or hCG-secreting tumor. Levels of hCG may take as long as 60 days to return to zero after an abortion.23 Failure to decline during 60 days and persistent elevation of hCG beyond 60 days after abortion may indicate an incomplete abortion, a twin pregnancy with only one fetus removed, or an ectopic pregnancy.1 The hCG assay continues to be the standard for the diagnosis of pregnancy. Routine clinical detection of pregnancy by ultrasonography is not possible until at least 5 weeks’ gestation, when the hCG level is 1000 mIU/mL or more.24–26 Ultrasonography is reliable for the diagnosis of intrauterine pregnancy during the first trimester. Systematic reviews demonstrate that ultrasonography scans performed in the ED have a sensitivity of 90% and specificity of 98% in the detection of an intrauterine pregnancy.27 In addition to diagnosis, it also locates the embryo and assesses gestational age and viability. Transabdominal sonography is more commonly used; however, transvaginal sonography may be necessary during the first trimester if transabdominal sonography is not diagnostic. Sonography during the second trimester is used to survey fetal anatomy. The anatomic survey includes the cerebral ventricles, four-chamber view of the heart, spine, stomach, urinary bladder, umbilical cord insertion on the abdominal wall, and kidneys.28 Much debate exists about whether ultrasonography should be performed as part of routine prenatal screening in all pregnancies. The American College of Obstetricians and Gynecologists (ACOG) recommends that all physicians discuss the benefits and limitations of ultrasonography with patients and come to a decision together about the screening examination.28 Although prenatal screening is still an object of contention, the ACOG states that there is good evidence for the safety and accuracy of ultrasonography examination to determine gestational age, major fetal anomalies, fetal number, viability, and placental location and recommends the test if these areas are in question.28 First-trimester ultrasonography, in conjunction with biochemical parameters, is an integral part of first-trimester genetic screening for aneuploidy and is usually performed at 11 to 13 weeks of age. Transabdominal Sonography.: At 6 weeks the gestational sac can be visualized by transabdominal sonography (Fig. 177-5). This sonographic finding disappears after the 11th week of gestation. Presence of the gestational sac is confirmed when two central uterine echoes are visualized within the hypertrophied endometrium. This diagnostic echo is called the double ring sign. The gestational sac is eccentrically positioned within an asymmetrically thickened decidua. A healthy pregnancy can be determined by correlating the size of the sac with gestational age and quantitative hCG values. At 8 weeks the fetal pole and fetal heart activity can be visualized reliably with transabdominal ultrasonography in a normal pregnancy. Diagnosis of an intrauterine pregnancy with ultrasonography can be made with the following findings: yolk sac or fetal pole visualized inside myometrium, bladder-uterine juxtaposition confirmed, and myometrial mantle of 5 mm29 (Fig. 177-6). Diagnosis of a live intrauterine pregnancy also requires visualization of intrauterine fetal heart activity. Transvaginal Sonography.: Transvaginal sonography affords greater resolution than transabdominal sonography and does not require a full bladder. Transvaginal ultrasonography examination can identify an intrauterine gestational yolk sac at 4 to 5 weeks’ gestation. Fetal heart motion can be detected with transvaginal ultrasonography evaluation at 6 weeks’ gestation. An intrauterine pregnancy can be diagnosed by sonographic findings in the following order of appearance: the double ring sign, the double gestational sac, the intrauterine fetal pole, and intrauterine fetal heart activity (see Fig. 177-6). No significant side effects have been documented to date with the magnetic field and radio waves used in magnetic resonance imaging (MRI). Gadolinium is the most common intravenous contrast agent, and allergic reactions with this agent are rare. Currently, the ACOG recommends that nonionizing imaging procedures like MRI and ultrasonography be used preferentially, when appropriate, and they are not known to be associated with adverse fetal effects.30 The decision to perform imaging investigations in a pregnant patient must take into consideration the risk to the developing fetus from radiation exposure and the inherent risks with such investigations for the mother, balanced with the risk to the mother and fetus associated with missing the diagnosis because of reluctance to use available diagnostic imaging techniques. The potential for adverse outcomes with intrauterine fetal exposure to the radiation used in radiography is minimal.31 Case-control studies report a slight but statistically significant increase in the relative risk for childhood cancer.32 Radiation is a dose-dependent teratogen.33 The fetal central nervous system is most vulnerable to the teratogenic effects of radiation at 8 to 15 weeks after conception. The fetal dose of radiation is estimated from the ovarian or uterine dose. Pregnant women exposed to less than 50 mGy have pregnancy outcomes similar to those of control subjects.34 A study of childhood cancers in the offspring of female radiologists revealed a cancer frequency of 0.16% in children exposed to 10 mGy of radiation in utero. In comparison, the frequency of cancer is 0.07% among children who were not exposed to radiation in utero. Table 177-1 summarizes the estimated fetal radiation exposure associated with the more common diagnostic imaging modalities. Radiation exposure depends on the equipment and technique, and estimates listed may not be generalizable to every radiology department. Table 177-1 Fetal Radiation Exposure with Various Imaging Modalities *Data from Duke University and Duke Medicine Radiation Safety Division: Fetal radiation dose estimates. Available at www.safety.duke.edu/radsafety/fdose/fdxray.asp. Accessed March 12, 2011. Modified from Ginsberg JS, et al: Thrombotic complications in the obstetric patient. In Coleman RW, et al (eds): Hemostasis and Thrombosis: Basic Principles and Clinical Practice, 3rd ed. Philadelphia, JB Lippincott, 1994.
General Approach to the Pregnant Patient
Perspective
Clinical Features
Physical Examination
Diagnostic Strategies
Radiology
Magnetic Resonance Imaging
Diagnostic Imaging Considerations in Pregnant Patients
PROCEDURE
ESTIMATED FETAL RADIATION EXPOSURE (µGy)
Chest plain film with an abdominal shield
10*
Chest CT with an abdominal shield
100*
Abdomen plain film
2400*
Abdomen CT: with and without contrast agent
20,000 and 10,000*
Cardiac catheterization: with and without pelvic fluoroscopy
13,000 and 1000*
Head CT
100*
Unilateral venography without an abdominal shield
3050
Limited venography with an abdominal shield
500
Pulmonary angiography via femoral access
4050
Pulmonary angiography via brachial access with an abdominal shield
500
Perfusion lung scan using 99mTc-MAA
3 mCi
180
1-2 mCi
60-120
Ventilation lung scan using
133Xe
30-200
99mTc-DTPA
70-350
99mTc-SC
10-50
Ventilation-perfusion scan
500

Full access? Get Clinical Tree


General Approach to the Pregnant Patient
Only gold members can continue reading. Log In or Register to continue