Chapter 87 Gastrointestinal Pharmacology
Nausea and Vomiting
Introduction and Definitions
Uncontrolled nausea and vomiting in the pediatric intensive care unit may result in consequences that range from minor patient discomfort to life-threatening infection or electrolyte imbalance. To the patient this can mean wound dehiscence, gastrointestinal (GI) bleeding, malnutrition, dehydration, pulmonary aspiration, and increased anxiety with future medical interventions.1 Indeed, nausea and vomiting has been referred to as the “big little problem” with the capacity to delay discharge, monopolize nursing care, and impact financial resources.2 These costs can be limited with a focus on prophylactic treatment, employing recent drug developments and guideline-driven therapy when possible. The data that drive these guidelines are, particularly in pediatrics, largely based on studies that look at vomiting more specifically than nausea.3 Because nausea is an unpleasant, largely subjective sensation in the epigastrium that may be associated with vomiting, it is more difficult to quantify objectively.3,4 Vomiting, defined as the forceful expulsion of gastric contents from the mouth, can be more easily measured for medication assessment purposes. Although distinct, when vomiting is discussed here, it can be inferred to also pertain to managing nausea (unless otherwise noted).
Pathophysiology
The vomiting center or emetic center or “central pattern generator,” located in the medulla oblongata, is a collection of neurons that may trigger vomiting after receiving input from the chemoreceptor trigger zone, abdominal vagal afferents or cerebral cortex.1,5–7 The chemoreceptor trigger zone, located in the area postrema region near the fourth ventricle, is not fully protected by the blood-brain barrier; and is therefore vulnerable to antineoplastic agents, or other stimuli in the blood or cerebral spinal fluid.5–7 The vagus nerve provides important stimuli to both the chemoreceptor trigger zone and vomiting center, and appears to have the largest role in CINV.5,7 This occurs when cells within the GI tract are exposed to chemotherapy and release mediators, including 5-hydroxytryptamine (5-HT), substance P, and cholecystokinin, that stimulate the vagus nerve.5,7 Finally, the role of the cerebral cortex is less well defined, but is thought to play a role in anticipatory nausea and vomiting.1
The mechanism described is most dependent upon the neurotransmitter receptors for dopamine, serotonin (5-hydroxytryptamine type 3), and substance P.1,5–7 Additional receptors that are thought to play a supporting role include corticosteroid, histamine, cannabinoid, acetylcholine, opiate, neurokinin-1, and gamma-aminobutyric acid.1,6,8 The intricate cascade briefly described here and multiple neurotransmitter involvement, foreshadow treatment guidelines that will often be rooted in multiple agents that target various pathways.
Chemotherapy-Induced Nausea and Vomiting: Types of Emesis
Emesis that occurs from chemotherapy is divided into three distinct categories. Anticipatory CINV occurs before chemotherapy administration and occurs as a conditioned response in patients that have had significant past CINV. Acute CINV occurs within 24 hours of chemotherapy administration and is the most widely studied. Delayed nausea and vomiting occur after 24 hours and may persist for 7 days. Pediatric patients that have well-controlled acute nausea and vomiting are less likely to have delayed nausea and vomiting.8 This highlights the importance of “staying ahead” of symptoms with prophylactic treatment.
Treatment Guidelines
The advent of 5-HT3 receptor antagonists in the early 1990s provided a new cornerstone in how acute nausea and vomiting is managed in patients receiving highly or moderately emetogenic chemotherapy. The first-generation 5-HT3 receptor antagonists of dolasetron, granisetron, and ondansetron have been established in pediatrics to be equally effective, and equally toxic, with the most common side effects including headache, constipation, and dizziness.3,9–13 It should be noted that the approved pediatric dose of granisetron of 10 ug/kg once daily is likely ineffective, and a dose of 40 ug/kg once daily is more appropriate.3,13,14,20 The 5-HT3 receptor antagonists have been established, at the appropriate doses, to be as effective when given orally as when given intravenously, and that single daily dose schedules are as effective as multiple-dose schedules.15–20 These agents are most useful for CINV occurring within the first 24 hours after administration of chemotherapy.21
In 2003, what may be considered a “second-generation” 5-HT3 receptor antagonist, palonosetron, gained Food and Drug Administration approval for both acute and delayed CINV. It is unique from other 5-HT3 antagonists because of its long half-life (21 to 37 hours in children and 40 hours in adults) and greater affinity for 5-HT3 receptors.14,22 Three phase III trials have established palonosetron as not inferior to older 5-HT3 antagonists.23–25 A trial comparing palonosetron with ondansetron in pediatric patients found a significant reduction in emesis on the first 3 days after treatment, and in nausea intensity the first 4 days after treatment favoring the palonosetron group.26 Further prospective study is needed before palonosetron can be established as superior to other 5-HT3 receptor antagonists. To date, CINV guidelines have not stated any one 5-HT3 receptor antagonist to be superior to another.20
Corticosteroids, most commonly dexamethasone or methylprednisolone, are potent antiemetics and have a critical role in managing acute and delayed CINV in pediatrics. Their exact mechanism of action is unclear, but may involve decreasing 5-HT3 release in the periphery.27 However, corticosteroids also have the potential to decrease the effect of antineoplastic drugs on brain tumors, osteosarcomas and carcinomas.27 Dexamethasone may augment the ability of a number of carcinomas to resist both radiation and chemotherapy.3 Interferon or interleukin-2 may be less effective when used along with steroids.1 Corticosteroids may also impact the quality of brain tumor images generated by computed tomography or magnetic resonance imaging by altering the distribution of contrast media.3 In addition to these concerns, the usual side effects of steroids must be kept in mind, including: hyperglycemia, hypokalemia, anxiety, euphoria, and insomnia. It would be prudent to consult a patient’s chemotherapy protocol or an oncologist before initiating corticosteroids in a pediatric patient with a malignancy to verify the appropriateness of this approach.
The neurokinin-1 receptor antagonist is the most recent antiemetic class. These are effective for both acute and delayed nausea and vomiting in moderately and highly emetogenic chemotherapy. Such agents are approved in CINV only when used in conjunction with a 5-HT3 antagonist and a corticosteroid. Currently the only medications that represent this class on the market are oral aprepitant and intravenous fosaprepitant. They are generally well tolerated with diarrhea, fatigue, headache, and hiccups being common side effects.28–30 Both aprepitant and fosaprepitant are substrates, inhibitors and inducers of the cytochrome P450 enzymes 3A4 and 2C9.14 A thorough review of potential interactions for individual patients is merited. Most notable of these cytochrome interactions are: The effect of Coumadin (brand name form of the generic warfarin) on coagulation pathway (altered prothrombin time, International Normalized Ratio) may be reduced, the concentration of corticosteroids will be increased with a dose reduction needed when used for CINV with aprepitant, and a backup contraception method should be used for patients taking oral contraceptives.30 This class of medication does not have approval for patients younger than 18 years, nor are suggested dose modifications available.14 One study examined aprepitant dosed for young adults in patients ages 11 to 19 years weighing 43 to 105 kg.31 Aprepitant containing regimens performed comparably to non-aprepitant-containing regimens, but an increase in the number of cases of febrile neutropenia was noted in the aprepitant patients.31 Further prospective study is necessary before neutrokinin-1 receptor antagonists are prescribed in pediatric patients.
Other agents commonly seen in pediatrics for CINV include benzodiazepines, most often lorazepam, which is routinely employed for anticipatory nausea and vomiting at a dose of 0.04 to 0.08 mg/kg/dose (maximum dose, 4 mg).14 Diphenhydramine is anecdotally used in pediatric patients despite its absence from guidelines and reviews of CINV.3,20 Dopamine receptor antagonists, including phenothiazines (e.g., prochlorperazine), butyrophenones (e.g., droperidol), and benzamides (e.g., metoclopramide), cause a high incidence of dystonic reactions when used at high doses for several days and potentially oculogyric crisis.20,32 Cannabinoids (i.e., dronabinol) are limited by their lack of pediatric data, and their side effects, which include euphoria, sedation, depression, and hallucinations.14 A scopolamine patch may have some utility in patients older than 12 years that are able to wait up to 12 hours for relief.3 Generally speaking, these agents should only be considered for pediatric patients who do not tolerate first-line therapy alone.
Determining appropriate antiemetic therapy requires one to first evaluate the likelihood that a particular chemotherapy regimen will produce nausea or vomiting.20,33–35 This has been accomplished with literature and expert opinion and is summarized in Table 87-1, with subsequent dosing information provided in Table 87-2.14,20,33–40 This categorization is largely based on adult data, so it is vital to take individual patient parameters and CINV history into account. If a chemotherapy regimen is close to the next highest emetic risk category, it is better to be aggressive with antiemetic management.
Table 87–1 Antiemetic Selection for Chemotherapy Based on Emetogenicity of Frequently Encountered Single-Day Pediatric Chemotherapy
| Emetic Risk (Incidence of Emesis with no Prophylaxis) | Antineoplastic Agent∗ | Recommended Antiemetic |
|---|---|---|
| High (>90%) | ||
| Moderate (30%-90%) | ||
| Low (10%-30%) | ||
| Minimal (<10%) | Children and adults: 5-HT3 antagonist or dexamethasone only if needed |
5-HT3, 5-Hydroxytryptamine-3; NK-1, neurokinin-1.
∗ Table focuses on more common pediatric chemotherapy (see references 20 and 33 through 38 for complete list). Medication is parental unless otherwise stated.
Table 87–2 Antiemetic Dosing in Chemotherapy-Induced and Postoperative Nausea and Vomiting14,36,39,40
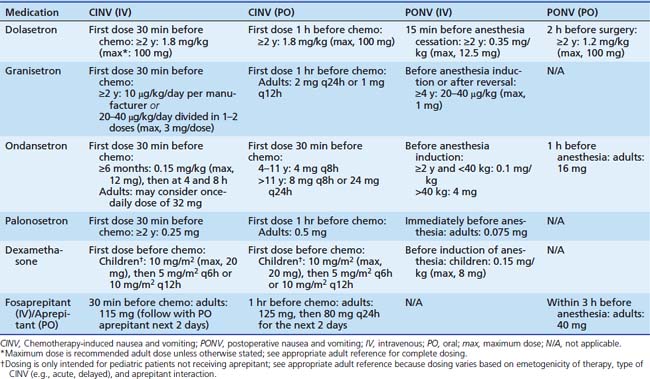
Table 87-1 includes aprepitant and dexamethasone that may be contraindicated for multiple reasons in children as discussed previously. In such patients, medications that are less-well supported for delayed CINV, such as diphenhydramine or 5HT3 receptor antagonists, may be employed based on individual practitioner preference. Other areas of future research include breakthrough emesis that occurs despite adequate prophylaxis in a single cycle, and refractory emesis that occurs despite adequate prophylaxis over multiple cycles.8 Additional areas that require further investigation in pediatric CINV include multiple-day chemotherapy, high-dose chemotherapy with stem cell rescue, and intractable symptoms in terminal patients.41–43 Finally, each institution would be well advised to conduct a pharmacoeconomic review to aid in antiemetic selection of pharmacologically equivalent products.
Postoperative Nausea and Vomiting
Assessing the risk of the pediatric surgical candidate is the first step in determining PONV prophylaxis. Strabismus repair, tonsillectomy, hernia repair, orchiopexy, penile surgery, or any procedure where anesthesia time is 30 minutes or longer have an increased likelihood of PONV.4,40,44 Children aged 3 years and older continue to increase their risk of PONV until they become pubescent.40,44 Patients who have a history of PONV, motion sickness, or patients with first-degree relatives with such histories are more prone to have PONV.40,44 Prophylaxis is recommended only for children thought to be at moderate or high risk of PONV.4 Regional anesthesia is optimal in such patients.4 However, if general anesthesia is necessary, the following may reduce the risk of PONV: using intraoperative supplemental oxygen, adequate hydration, minimizing opioid or neostigmine exposure, and avoiding nitrous oxide or volatile anesthetics when possible.40
Individual institutions should determine which patients are at moderate or high risk. These patients should receive a combination of two or three agents with distinct mechanisms of action for PONV prophylaxis.4,40 A 5HT3 receptor antagonist and dexamethasone combination is a reasonable first choice.4 Other options include dimenhydrinate, perphenazine, or droperidol.4,40,44 Because of potential for QT prolongation and torsades des pointes, droperidol should be reserved for patients who have failed first-line therapy and are hospital inpatients.4 Droperidol should be dosed once for prophylaxis at 0.05 mg/kg (maximum, 1.25 mg), or at 0.01 to 0.03 mg/kg/dose for PONV treatment.14,40 Finally, NK-1 receptor antagonists have been established as potent agents for PONV in adult patients, but even if approved in pediatrics it seems likely a multiple agent approach to PONV as described above will remain the standard of care.45–48
Diarrhea
Introduction and Definition
It is estimated that in the United States, diarrhea-based illnesses result in approximately 200,000 hospital admissions and 400 deaths per year.49 The majority of acute diarrhea episodes stem from infections to the GI tract and it is estimated that 20% to 40% of children treated with broad-spectrum antibiotics will develop diarrhea.50,51 Infectious causes include viruses (e.g., Rotavirus), bacteria (e.g., Escherichia coli, Shigella, Clostridium difficile), and parasites (e.g., Cryptosporidium parvum, Giardia lamblia). Noninfectious causes include appendicitis, intussusception, food allergies, irritable bowel syndrome, and ulcerative colitis. Diarrhea may be defined for practical purposes as a decrease in stool consistency, along with an increase in frequency to more than three bowel movements in 24 hours.49 After diarrhea is identified, rehydration is paramount. Identifying the underlying cause will guide specific treatment.
Treatment
If C. difficile is identified, any potentially contributing broad-spectrum antibiotics should be discontinued, or streamlined to more a specific agent if possible. Oral metronidazole for 10 days is first line therapy in mild to moderate disease.51,52 Cases that require protracted courses of metronidazole should include monitoring for peripheral neuropathy. Oral vancomycin should be considered for patients with severe symptoms, intolerance to metronidazole, or metronidazole failure.51–53 Indeed, there is growing evidence in the adult population of superior outcomes when vancomycin is used first line for severe disease.52,53 Probiotics, bile-acid sequestrants, intravenous immunoglobulin, or tapering doses of vancomycin may be considered for recurrent cases. The impact of C. difficile can be minimized with prevention that includes good hand hygiene, infection control, and appropriate antibiotic usage. It would be prudent to limit acid suppressing agents (e.g., proton pump inhibitors, histamine H2 antagonists) to legitimate indications, as a positive association has been identified in adult populations between acid suppression and C. difficile disease.54,55
Probiotics are widely defined as live microorganisms that offer health benefits. These agents have been studied in pediatric patients in terms of potential reduction in antibiotic-associated diarrhea. One meta-analysis found that for every seven pediatric patients administered probiotics, one fewer would develop antibiotic-associated diarrhea. Positive risk reduction was identified, in decreasing strength, for those treated with Lactobacillus GG, Saccharomyces boulardii, and the combination of Bifidobacterium lactis and Streptococcus thermophilus.56 Significant reductions were not found with L. acidophilus/Bifidobacterium infantis or L. acidophilus/L. bulgaricus.56 A second meta-analysis reviewing Lactobacillus GG, L. sporogenes, and Saccharomyces boulardii found a significant decrease in antibiotic-associated diarrhea but nonsignificant overall results in intent-to-treat analysis.57 Such agents require further study from an efficacy and pharmacoeconomic standpoint before they become the standard of care. If these agents are used, sufficient live organism content should be verified. These agents should be used cautiously, if at all, in premature infants and in patients with any of the following: short bowel syndrome, central catheters, cardiac valve disease, and immune compromise.58,59
Other important treatments for diarrhea include the antimotility agents loperamide and diphenoxylate. Both inhibit excessive GI peristalsis and increase GI transit time.14 Neither medication should be used in diarrhea resulting from pseudomembranous enterocolitis or any infectious cause, because it is critical that toxins produced are cleared from the GI tract.14,49 Underlying electrolyte imbalances and dehydration should be addressed before these antimotility agents are initiated, because the potential exists that intestinal fluid retention may exacerbate such underlying conditions. Side effects to be mindful of include: sedation, confusion, ileus, and respiratory depression (with younger children at increased risk).14
Constipation
Introduction and Definition
Constipation is defined as a reduction in the number of bowel movements. This number varies significantly based on the infant or child’s age and breastfeeding status with exclusively breast-fed infants having an increased frequency of bowel movements.60,61 The differential diagnosis underlying constipation in a pediatric intensive care unit is extensive and includes: anatomical (e.g., anal stricture, rectal abscess), metabolic (e.g., hypothyroidism, cystic fibrosis), neurologic (e.g., Hirschsprung disease, cerebral palsy), and connective tissue disorder (e.g., systemic lupus erythematosus).61 Medications such as opiates, phenobarbital, antacids, antihypertensives, anticholinergics, and antidepressants, and lead may all contribute to a child’s constipation.61
Treatment
Any underlying disease state or anatomical anomaly should be managed individually. If medication related, the offending agent should be discontinued if possible; or the minimal effective dose should be targeted. Constipation itself is addressed as a two-step process of disimpaction lasting 3 to 5 days, followed by maintenance. The duration of the latter stage is determined by symptom recurrence when treatment is withdrawn.60,62
Disimpaction may be accomplished with oral or rectal medication administration. The oral route is less invasive and may give a sense of empowerment to the child, but the rectal route is quicker and may be necessary if the child has abdominal pain. Oral options include mineral oil, oral electrolyte solutions, lactulose, sorbitol, magnesium hydroxide, magnesium citrate, senna, and bisacodyl. Mineral oil may result in lipoid pneumonia if aspirated and is not recommended in children younger than 1 year or any patient on aspiration precautions. Options for rectal disimpaction include phosphate soda, saline, or mineral oil enemas followed by a phosphate enema. Also effective are glycerin suppositories in infants, and bisacodyl suppositories in older children. Soapsuds, tap water, and magnesium enemas are discouraged because of their potential toxicity.60 Enemas are to be avoided in children younger than 3 years and in young children with severe neurologic deficiencies as they are more likely to be retained with subsequent resulting toxicities.62
Maintenance therapy with polyethylene glycol 3350 without electrolytes (MiraLax) has demonstrated superior palatability in children, and although not yet recommended by guidelines in infants, there is growing evidence of its safe use in this population.60,62 Other maintenance therapies include mineral oil, magnesium hydroxide, lactulose, and sorbitol. Bisacodyl or senna may be necessary as a stimulant laxative as rescue therapy, but should be avoided for extended use.60
Gastroesophageal Reflux Disease
Treatment of gastroesophageal reflux disease (GERD) should alleviate symptoms and prevent or heal esophageal damage. Although researchers conducting multiple pediatric studies have used varying doses, multiple drug therapies, and non-pharmacologic therapies, guidelines do exist for the treatment of GERD in infants and children.63 Many patients who may be admitted to the intensive care unit for other conditions are likely to have preexisting gastroesophageal reflux conditions and corresponding surgical and pharmacologic therapies. These populations include patients with reactive airway disease, recurrent pneumonia, neurologic impairment, premature infants, and genetic syndromes including Down syndrome.
Histamine-2 Receptor Antagonists
Histamine-2 (H2) receptor antagonists inhibit gastric acid secretion by means of competitive inhibition of H2 receptors of the gastric parietal cells. H2 receptor antagonists are generally well tolerated. Common adverse effects include nausea and headache. Although thrombocytopenia may occur, it is often difficult to determine the sole cause in critically ill patients. Ranitidine should be avoided in patients with acute porphyria. Tachyphylaxis is well documented for intravenous and oral H2 receptor blockers in adult and pediatric populations. Infants may suffer from irritability, headache, and somnolence, which when mistaken for GERD symptoms, may lead to an unwarranted increase in dose.63 All of the currently available H2 receptor blockers require dosage adjustment for renal impairment. Although fewer pediatric studies exist for ranitidine and famotidine, these agents are frequently used and may be preferred over cimetidine because of fewer drug interactions and central nervous system adverse effects. Availability of both oral and intravenous formulations allow for easy continuation of therapy when patients are admitted to the intensive care unit.
Proton Pump Inhibitors
By covalently bonding to the hydrogen-potassium-adenosine triphosphatase (ATP) of parietal cells, proton pump inhibitors (PPIs) suppress gastric acid secretion for the life of the parietal cell. Ideally, therapy should be given 30 minutes before feeding so that peak plasma concentrations coincide with parietal cell stimulation. Administration with food reduces bioavailability by fifty percent. Although their serum half-lives are relatively short; the pharmacological effect persists until new parietal cells are generated.64 Omeprazole, esomeprazole, and lansoprazole have been approved for use in pediatrics. Extemporaneously compounded suspensions of omeprazole and lansoprazole are frequently used, even in patients with various types of feeding tubes. Intravenous lansoprazole is no longer available. Neither intravenous pantoprazole nor esomeprazole is Food and Drug Administration (FDA)–approved for use in pediatric patients. Until sufficient pediatric dosing information becomes available, intravenous PPIs are likely to be reserved for patients with active GI bleeding or contraindication to alternative therapies. Although PPIs are generally thought of as well tolerated, both long- and short-term adverse effects are gaining more attention. Reactions include idiosyncratic reactions, drug-drug interactions, drug-induced hypergastrinemia, and drug-induced hypochlorhydria.63 The most common side effects are headache, diarrhea, constipation, and nausea.14 Case reports of biopsy proven interstitial nephritis associated with PPI use have emerged. Thus far no pediatric cases have been reported.63 The FDA and Health Canada are currently investigating potential links between PPIs and decreased effectiveness of clopidogrel. Although the data are primarily from adult reports, the potential interaction may be relevant in pediatrics as well. PPIs should not be discontinued abruptly because a hypersecretory phase results.63,65 Acid suppression, whether from H2 receptor antagonists or PPIs, may increase the rates of community-acquired pneumonia, gastroenteritis, candidemia, and necrotizing enterocolitis.63,66–68
Antacids
Both aluminum hydroxide and magnesium hydroxide are effective treatments of GERD. In infants, however, aluminum hydroxide formulations can increase plasma levels of aluminum to those reported to cause osteopenia, microcytic anemia, and neurotoxicity. Aluminum-based antacids should be avoided in patients with renal impairment. Efficacy of calcium carbonate antacids has not been demonstrated in pediatric patients. Depending on the antacid formulation chosen, adverse effects may include electrolyte disturbances, nausea, flatulence, diarrhea, or constipation. Because alternative therapies are available and generally well tolerated, antacids should be reserved for intermittent symptom management rather than chronic treatment of GERD.63
Surface Agents
Sucralfate, an aluminum salt of sulfated sucrose, forms a protective barrier by binding to damaged mucosa in an acidic environment. Although it has demonstrated effectiveness in the treatment of esophagitis, it is not recommended for long-term treatment of GERD in children because of a lack of efficacy data and safety concerns associated with aluminum toxicity.63
Prokinetic Therapy
Erythromycin is a macrolide antibiotic and motilin agonist. It increases motility in the proximal GI tract. The most frequent adverse effects include abdominal cramping, nausea, vomiting, diarrhea, cardiac dysrhythmias with interacting medications, and risk of bacterial overgrowth. Metoclopramide promotes gastric emptying by acting as an antagonist to the inhibitory actions of dopamine in the gut. It also sensitizes the gut to acetylcholine and increases lower esophageal sphincter tone.69 Metoclopramide blocks dopamine receptors in the chemoreceptor trigger zone and accelerates gastric emptying and intestinal transit time without stimulating gastric, biliary, or pancreatic secretions.14 Adverse effects are common; are dose dependent; and can include extrapyramidal effects, seizures, and Parkinsonian reactions, which may be irreversible.14,63 The FDA required the addition of a boxed warning to metoclopramide drug labels regarding the risks of long-term or high-dose use. Chronic use of metoclopramide has been linked to tardive dyskinesia, which may include involuntary and repetitive movements of the body, even after drug discontinuation. It is noted that these adverse effects are more prevalent in elderly populations.70 Extrapyramidal reactions in children and younger adults are more likely after intravenous administration of high doses, particularly within 24 to 48 hours after starting therapy.14 Prescribers are urged to carefully consider legitimate indications for initiating drug therapy. Current GERD guidelines recommend against use of prokinetic agents.
Gastroesophageal Reflux Disease and Acute Life-Threatening Event
An acute life-threatening event (ALTE) is an episode of combined apnea, color change (cyanosis, pallor, plethora), abnormal muscle tone (limpness and stiffness), choking, and gagging that requires intervention. Some patients have succumbed to sudden infant death syndrome who have had a history of ALTE with documented GER. This combination is rare and assigning the timing and causality of the event is extremely difficult. Neither pharmacotherapy nor surgical intervention has been adequately studied in secondary prevention of ALTE. Those patients who may benefit from pharmacologic intervention may include those whose ALTEs were obviously associated with vomiting, regurgitation, or obstructive apnea.63
Stress-Induced Mucosal Damage: Ulcer Prophylaxis
Prospective studies reveal similar incidence of GI hemorrhage between adult and pediatric intensive care units. Of 1006 patients admitted to a pediatric intensive care unit, 10.2% had upper GI hemorrhage; however, only 1.6% of the admissions were viewed as clinically significant. Respiratory failure, coagulopathy, and a Pediatric Risk of Mortality score greater than or equal to 10 were identified as independent risk factors for GI hemorrhage.71,72 Other studies also identified pneumonia, multitrauma, a surgical operation longer than 3 hours, and circulatory shock in patients as high-risk factors.73,74 One subsequent pediatric study demonstrated mechanical ventilation as the only statistically significant risk factor for clinically significant bleeds after multivariate analysis.75 Children ventilated for greater than 48 hours were at additional risk of hemorrhage if thrombocytopenia, coagulopathy, organ failure, high pressure ventilator settings (>25 cm H2O), or Pediatric Risk of Mortality score greater than 10 were present.76 Mechanical ventilation and coagulopathy are recognized as independent risk factors in adult patients.72 Because of the low incidence of clinically significant hemorrhage, prophylaxis may only be warranted in those patients with risk factors.77
Sucralfate, H2 receptor antagonists, antacids, and PPIs have been used for prophylaxis of stress-induced mucosal damage in pediatric critical care patients. Individual trials including comparative studies and case series have shown each agent to be effective. With the low incidence of clinically significant bleeds, studies intending to demonstrate superiority of one drug class or agent over another would require an extremely large sample size. Therefore pediatric studies use a surrogate endpoint of gastric pH 4 or greater to evaluate efficacy of various dosing regimens.78–80
One study included 165 patients treated with ranitidine, antacid, sucralfate, or placebo. There were no differences among the treatment groups and all treatment groups had a lower incidence of bleeding than the placebo group.81 Another study compared ranitidine, omeprazole, sucralfate, or placebo for stress ulcer prophylaxis and also compared adverse events. No differences were found among groups with regard to macroscopic stress ulcer bleeding, mortality, or ventilator-associated pneumonia.82 Because multiple agents appear to be effective for stress ulcer prophylaxis, selecting an appropriate agent may be patient specific. However, when prescribing prophylaxis to adult patients, the inadequacy of sucralfate it is widely recognized after the landmark trial by Cook et al.83 published in 1998 that demonstrated significantly lower rates of clinically relevant bleeding in the ranitidine treatment group. In addition there was no significant difference in pneumonia rates in this 1200-patient trial. Critical care patients do present challenges with regard to drug administration for antacids and sucralfate. Both agents require multiple doses per day, lack intravenous formulations, and may chelate or inhibit absorption of other drugs. In addition, aluminum toxicity may be of greater concern in critical care patients because of the potential for renal impairment. H2 receptor antagonists are commonly used because of the availability of intravenous formulations. Adverse effects of the central nervous system, particularly in patients with renal impairment who have not been dose adjusted; thrombocytopenia; and drug interactions remain a concern. Most of these adverse events, however, can be prevented or at least monitored for early detection. PPIs may also be used orally, intravenously, or off-label through feeding tubes.
Stress ulcer prophylaxis is recommended, along with other supportive therapies, for acute lung injury and acute respiratory distress syndrome in children.84 Stress ulcer prophylaxis is also recommended as supportive care for patients with sepsis in the 2008 Surviving Sepsis Campaign. H2 receptor antagonists use was recommended with a stronger level of evidence than PPIs.85 Some experts believe that although greater acid suppression is achieved with PPIs over H2 receptor antagonists, PPIs should be used as second-line agents for stress ulcer prophylaxis because of the greater association with adverse effects such as community-acquired pneumonia, ventilator acquired pneumonia, and C. difficile infection.86

Full access? Get Clinical Tree







