CHAPTER 21
Gastroenterologic Cancers
Catherine M. Concert, DNP, RN, FNP-BC, AOCNP, NE-BC, CGRN
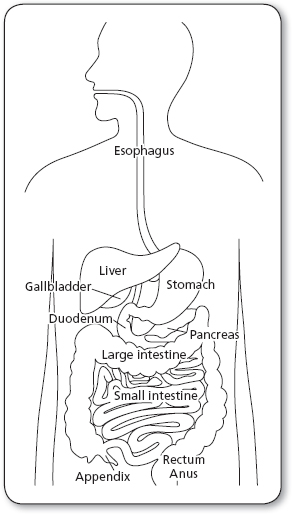
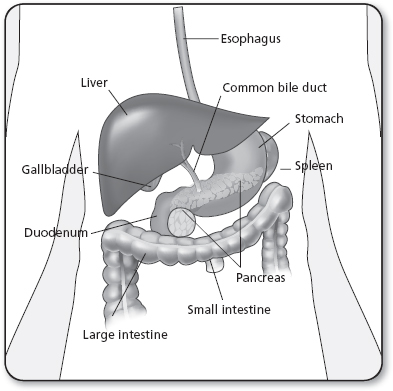
Cancers of the gastrointestinal (GI) system (Figure 21.1) are malignancies that include the esophagus, stomach, small and large intestines, rectum, anus, and the biliary tract, incorporating the liver, gallbladder, and pancreas.
 ESOPHAGEAL CANCER
ESOPHAGEAL CANCER
Cancer of the esophagus is a rare but aggressive malignancy, the etiology of which remains uncertain. Most patients present with locally advanced disease and have a poor overall survival rate and diminished quality of life. In 2013, it was estimated that 17,990 people will be diagnosed with esophageal cancer in the United States (National Cancer Institute [NCI], 2013a). Approximately 95% of patients who have esophageal cancer will die from it, and 75% of patients will die within 1 year of diagnosis. Efforts to lower mortality rates in the United States have so far been disappointing; esophageal cancer could be treatable but it is rarely curable. Despite many new and innovative approaches to the treatment of esophageal cancer, the overall 5-year survival rate in patients with definitive treatment ranges from 5% to 30%, (NCI, 2013a). Screening the population at large for early and perhaps treatable esophageal cancer is difficult and impractical. Nevertheless, epidemiologic data suggest that the incidence of esophageal cancer can be greatly reduced if environmental risk factors are controlled. In addition, identifying patients at risk of this disease increases the likelihood that patients will present with earlier, more favorably staged disease.
Anatomy, Physiology, and Pathology
The esophagus is a muscular tube, approximately 18 to 26 cm long, that extends from the level of the 6th cervical to the 11th thoracic vertebra. It has no significant secretory or absorptive functions and works solely to transport swallowed material from the pharynx to the stomach. In its mediastinal course, the esophagus is closely related to the trachea, bronchus, pulmonary veins, pericardium, and left atrium anteriorly, the pleura and lungs laterally, and the vertebral column and thoracic aorta posteriorly. Separating the esophagus from the vertebral column are the thoracic duct, azygos and hemiazygos veins, and posterior intercostal arteries. Unlike the remainder of the GI tract, the esophagus has no serosa. It is separated from adjacent structures by only a loose connective tissue that provides little barrier to the local spread of tumor.
For convenience, the esophagus is often divided into upper, middle, and lower thirds. The upper third extends to the aortic arch, the middle third to the inferior pulmonary vein, and the lower third to the esophagogastric junction.
Cancer of the esophagus has a propensity for rapid invasion of the esophageal wall and easy, widespread dissemination by way of a rich lymphatic supply. In general, lymphatic metastases involve the regional nodes closest to the site of primary tumor. However, because of the rich anastomoses of intramural lymphatic channels, nodal involvement may occur at substantial distances from the primary lesion.
Distant metastases can be found anywhere throughout the body. The liver, lungs, pleura, and kidneys are the most common sites, but the adrenal glands, bone, brain, heart, and peritoneum can be involved. Occasionally, the tumor may extend directly into mediastinal structures before distant metastasis is evident.
Pathology
SQUAMOUS CELL CARCINOMA
Squamous cell carcinoma is the second most common tumor of the esophagus, accounting for less than half the esophageal cancers in the United States (American Cancer Society, 2013). Unless detected in its earliest stages, it is usually fatal in <5 years. Histologically, the tumor is composed of sheets of polygonal or polyhedral cells with varying degrees of differentiation. Well-differentiated tumors contain features such as keratin pearls and intercellular bridges, whereas poorly differentiated tumors have marked nuclear and cellular pleomorphism. The majority of tumors, however, are moderately differentiated. Macroscopically, 60% of these lesions are fungating intraluminal growths, 25% are ulcerative lesions associated with extensive infiltration of the adjacent esophageal wall, and 15% are infiltrating. Most tumors are located in the distal part of the esophagus (NCI, 2013a).
ADENOCARCINOMA
Adenocarcinoma has been on the rise in the last two decades, and is the most common form of esophageal cancer in the United States, accounting for more than 50% of all new cases (Brown, Devesa, & Chow, 2008). Esophageal adenocarcinoma can arise at any level but is located most often at or near the gastroesophageal junction. Histologically, various degrees of glandular differentiation are noted, but well-differentiated tumors predominate. Esophageal adenocarcinoma is typically flat or ulcerated, although about one third of lesions are polypoid or fungating.
OTHER MALIGNANCIES
Several other rare types of esophageal malignant tumors occur, and all have a poor prognosis. Anaplastic small cell (oat cell) carcinoma accounts for fewer than 2% of esophageal cancers (Huang et al., 2013). Like their pulmonary counterparts, they arise from the amine precursor uptake and decarboxylation (APUD) cell system and demonstrate neurosecretory granules on electron microscopy. Malignant melanoma is exceedingly rare and constitutes <0.1% of esophageal malignancies (Blay, Le Cesne, Cassier, & Ray-Coquard, 2012). The tumor is more common in men and is clinically indistinguishable from other esophageal neoplasms. Adenoid cystic carcinoma typically occurs as a middle-third esophageal tumor and is usually discovered late in its course. Carcinosarcoma of the esophagus (also known as pseudosarcoma, spindle cell carcinoma, or polypoid carcinoma) is a tumor with histological features of both squamous cell carcinoma and malignant spindle-cell sarcoma. Found primarily in the distal two thirds of the esophagus, it can grow large (10–15 cm; Lokesh, Naveen, & Pawar, 2010; Sadej, Feld, Toll, & Palazzo, 2011). Sarcomas of the esophagus are rare, but a number of variants have been described. These include leiomyosarcoma, fibrosarcoma, rhabdomyosarcoma, liposarcoma, and malignant mesenchymoma. Primary malignant lymphoma has been seen in patients having HIV/AIDS. Esophageal lymphoma has assumed greater significance and should be evaluated as a differential diagnosis for immunosuppressed patients presenting with esophageal symptoms (Ghimire, Wu, & Zhu, 2011). Other types of benign esophageal tumors include leiomyomas, squamous cell papilloma, granular cell tumor, inflammatory pseudotumor, adenomas, hemangioma, neurofibroma, schwannoma, rhabdomyoma, lipoma, choristoma, amyloid tumor, and hamartoma. Finally, involvement of the esophagus by another primary tumor, either by direct extension or metastatic spread, has been reported with cancers of the breast, lung, liver, kidney, prostate, and stomach.
Epidemiology
There is wide variation in the incidence of esophageal cancer throughout the world. Worldwide, about 400,000 new cases of esophageal cancer are diagnosed annually; it is the eighth most common cancer and the sixth most common cause of cancer-related mortality (Jemal et al., 2011). The disease is uncommon in most of Western Europe and the United States. Clusters of high incidence occur in certain parts of Asia and Africa, which have the highest mortality rates (Jemal et al., 2011). Squamous cell carcinomas are the most common type and endemic in parts of China, Central Asia, Iran, Puerto Rico, and Africa. The incidence of esophageal squamous cell carcinoma is relatively low in the United States; more than half of all newly diagnosed cases of esophageal cancer in the United States are adenocarcinomas. The United Kingdom has the highest incidence of and mortality rate due to esophageal adenocarcinoma (Eslick, 2009). This variation, which cannot be explained by differences in reporting, indicates a great geographic range in etiological factors for this cancer.
By comparison, esophageal cancer is relatively uncommon in North America, Australia, and most European countries. Squamous cell carcinoma is more common, with higher incidence and death rates among African Americans than Whites; squamous cell carcinoma of the esophagus is the seventh leading cause of cancer death in African Americans, whereas 95% of adenocarcinomas affect mostly White men (Eslick, 2009).
Since the mid-1970s, tumor registry data obtained from the Surveillance, Epidemiology, and End Results (SEER) program have shown a decrease in the incidence of squamous cell carcinoma of the esophagus and an increase in adenocarcinoma of the esophagus in the United States; there was an average increase of 1.7% in men and 1.9% in women per year from 1999 to 2008 (Siegel, Naishadham, & Jemal, 2012). There were an estimated 17,990 new cases and 15,210 deaths from esophageal cancer in the United States in 2013 (NCI, 2013a). Esophageal cancer is three to four times more common among men than women; it is estimated that 14,440 cases will occur in men and 3,550 will occur in women. Esophageal cancer incidence increases with age and <15% of these cancers occur in those under the age of 55 years (Eslick, 2009). Most of these tumors arise in the lower third of the esophagus in the sixth decade of life.
Etiology and Risk Factors
The etiology of esophageal cancer remains unknown, but a number of factors, such as environmental exposure, dietary habits, chronic mucosal irritation, infection, cultural influences, and genetic predisposition, have been implicated (Table 21.1). A complex interaction among these risk factors probably exists, and their effects may be additive or even synergistic.
Several epidemiological studies implicate tobacco use and alcohol consumption as predisposing factors in the development of squamous cell carcinoma of the esophagus, especially in the United States and Western Europe (Kamangar, Chow, Abnet, & Dawsey, 2009). The risk from alcohol is directly related to the quantity and the type of alcohol ingested, with hard liquor posing a greater threat than wine or beer (Vioque et al., 2008).
Although cigarettes have received the most attention, tobacco can be carcinogenic in any form. Pipe tobacco, cigars, snuff, and chewing tobacco all increase the risk (Kamangar et al., 2009; Pelucchi, Gallus, Garavello, Bosetti, & La Vecchia, 2008; Vioque et al., 2008). The precise mechanism of tobacco carcinogenesis has not been fully characterized, but smoke constituents such as nitrosamines presumably play a role. In general, smokers run a risk four times greater than nonsmokers of developing esophageal cancer (Tramacere, La Vecchia, & Negri, 2011).
Multiple risk factors for the development of adenocarcinoma of the esophagus have been proposed. Unlike squamous cell carcinoma, there is no evidence that tobacco, alcohol, or diet plays a major pathogenic role in the development of esophageal adenocarcinoma. The only known predisposing factor is Barrett’s mucosa (metaplastic columnar epithelium), with severe dysplasia developing from chronic gastroesophageal reflux. Patients with a columnar-lined lower esophagus (Barrett’s metaplasia) are 40 times more likely to develop adenocarcinoma than the general population (Zhu et al., 2009).
Risk Factors Associated With Esophageal Cancer |
DIETARY |
N-nitroso compounds Alcohol (liquor > beer) Hot tea Tannins Vitamin deficiency (A, E, C, niacin, zinc) Retinol-containing foods Diet high in processed meats |
CHRONIC IRRITATION |
Tobacco Betel nut chewing Esophagitis (GERD, hiatal hernia, radiation) Strictures (lye, radiation) Injection sclerotherapy Barrett’s esophagus |
CHRONIC INFECTION |
Fungal Viral (HPV) |
MISCELLANEOUS |
Previous head and neck or lung malignancy Achalasia Plummer–Vinson syndrome/Paterson–Kelly syndrome Zollinger–Ellison syndrome Scleroderma Tylosis Celiac disease Previous gastrectomy Esophageal diverticula Obesity |
GERD, gastroesophageal reflux disease; HPV, human papillomavirus.
Source: Falk (2009).
Diagnostic Criteria
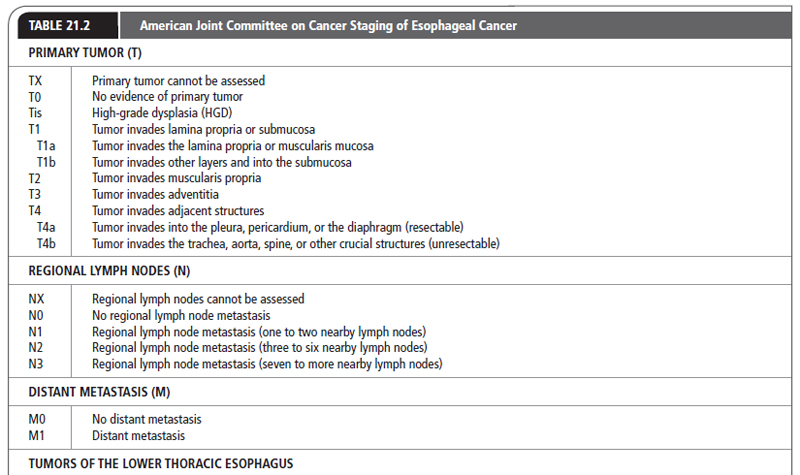
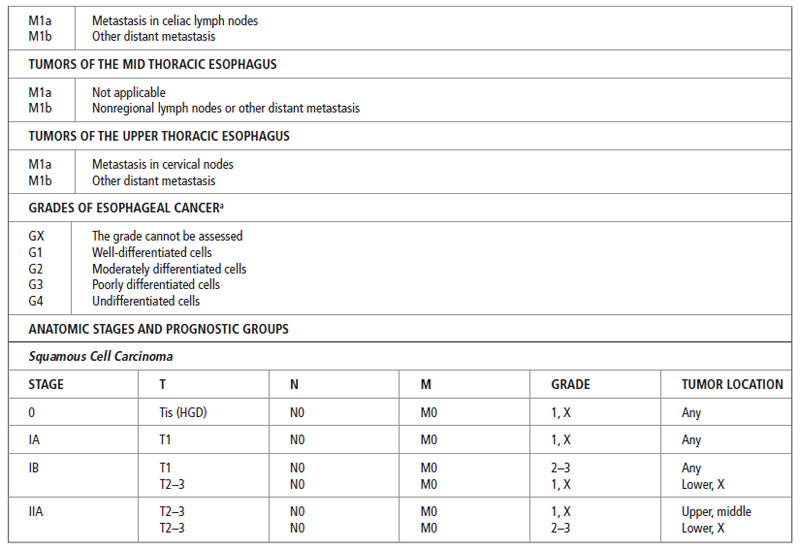
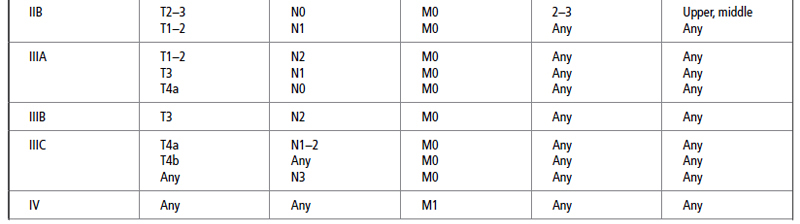
After a histological diagnosis is made, patients with esophageal cancer are best staged by the tumor–node–metastasis (TNM) system developed by the American Joint Committee on Cancer (AJCC). In this system, the esophagus is divided into four regions:
 Cervical, from the lower border of the cricoid cartilage to the thoracic inlet, approximately 18 cm from the upper incisor teeth
Cervical, from the lower border of the cricoid cartilage to the thoracic inlet, approximately 18 cm from the upper incisor teeth
 Upper thoracic, from the thoracic inlet to the tracheal bifurcation at approximately 24 cm from the incisors
Upper thoracic, from the thoracic inlet to the tracheal bifurcation at approximately 24 cm from the incisors
 Midthoracic, from the tracheal bifurcation to half the distance to the esophagogastric junction at 32 cm from the incisors
Midthoracic, from the tracheal bifurcation to half the distance to the esophagogastric junction at 32 cm from the incisors
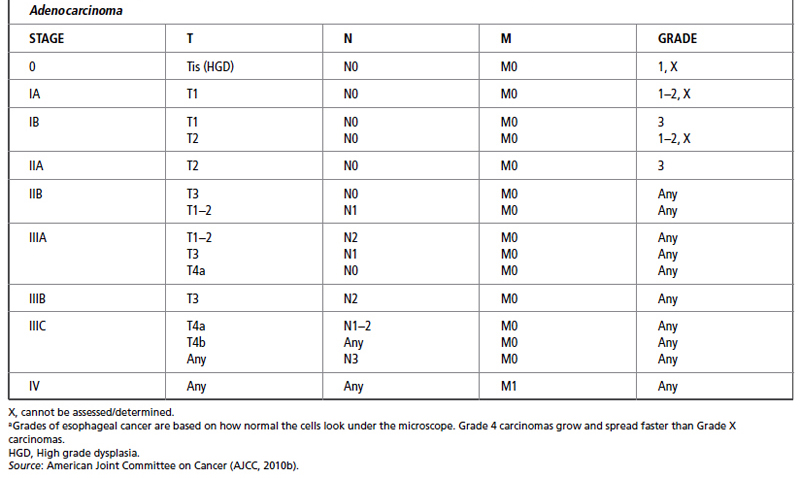
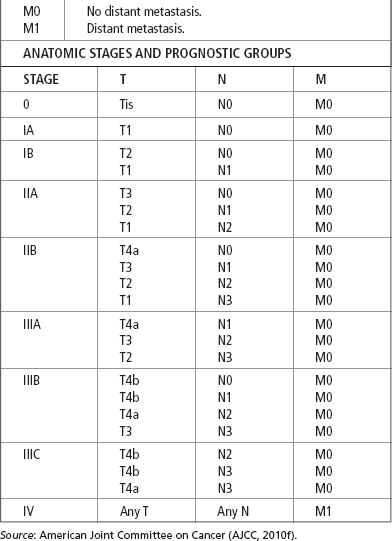
The most recent TNM classification and stage grouping is presented in Table 21.2.
In addition to stage, several aspects of tumor biology are associated with a poor prognosis. These include a poor histopathological grade, DNA ploidy status, and a high score on the argyrophilic nucleolar organizer regions test (AgNOR number; Redfield et al., 2013). Adenocarcinoma of the esophagus can have P53 mutations, aneuploidy, and microsatellite instability (MSI; Saeki et al., 2011). Anatomic location has also been found to have prognostic significance, with upper and midthoracic lesions having a less favorable outcome than other sites (AJCC, 2010b).
History and Physical Examination
HISTORY
Dysphagia and weight loss are the initial symptoms of cancer of the esophagus in 90% of patients (Perez & Brady, 2011; Table 21.3). Unfortunately, these are late symptoms in the natural history of the disease. Because of its distensibility, difficulty in swallowing does not occur until at least half the circumference of the esophagus has been infiltrated by cancer (Shimizu, Zaninotto, Nagata, Graham, & Lauwers, 2013). By this time, the tumor may have grown to a significant size, with local invasion or metastases. Occasionally, the onset of dysphagia is sudden, but most patients complain of an ill-defined retrosternal discomfort or a vague difficulty in swallowing for the preceding 3 to 6 months. Although most patients can localize the site of obstruction, this does not always correlate with the actual location of the tumor. Odynophagia (painful swallowing) occurs in more than 50% of patients and may be the only presenting symptom (Perez & Brady, 2011). Weight loss is common, but severe weight loss and cachexia are infrequently seen and are usually indicative of locally advanced or widespread disease. Patients may also experience regurgitation of undigested food, epigastric pain, chest pain, or aspiration pneumonia.
Advanced lesions may present with a variety of symptoms such as hematemesis, melena, superior vena cava syndrome, cough from a bronchoesophageal or tracheoesophageal fistula, hemoptysis, or problems related to nerve involvement (e.g., Horner’s syndrome or paralysis of the recurrent laryngeal or phrenic nerve; Jonker et al., 2009). Aortoesophageal fistula is a rare but lethal complication. Other signs of unresectable malignant disease may be found with malignant pleural effusion or malignant ascites.
PHYSICAL EXAMINATION
Apart from features of recent weight loss, the physical examination is often unremarkable, and the diagnosis of esophageal cancer will depend on radiographic or endoscopic data. However, the physical examination should encompass a thorough search for evidence of metastases. Supraclavicular or cervical lymph nodes should be sought and samples taken for biopsy if palpable. Enlargement of the left gastric, celiac, and retropancreatic nodes may be palpable in thin or cachectic patients, and there may be hepatomegaly in patients with liver metastases. Other evidence of intra-abdominal disease includes the presence of an epigastric mass, ascites, enlarged ovaries (Krukenberg tumors), or a Blumer’s shelf on rectal examination (Makis et al., 2012). Documentation of metastatic disease establishes the presence of a Stage IV tumor.
DISEASE COURSE
Squamous cell carcinoma of the esophagus is an aggressive tumor. It tends to infiltrate locally, involving adjacent lymph nodes and metastasizing widely by lymphatic or hematogenous routes. Lack of an esophageal serosal layer tends to favor local tumor extension into such structures as the pericardium, aorta, tracheobronchial tree, diaphragm, and left recurrent laryngeal nerve. Lymph node metastases are present in at least 75% of patients at the time of diagnosis, especially when tumors are 5 cm and larger (Perez & Brady, 2011). Distant spread to the liver and lung is common. The overall prognosis of invasive squamous cell carcinoma is poor, with slightly better prognosis for adenocarcinomas (American Cancer Society, 2013). As is the case with squamous cell carcinoma, adenocarcinoma of the esophagus exhibits aggressive behavior, with frequent transmural invasion and lymph node metastases. Distant spread is common, with the liver and lung most frequently involved. The 5-year survival rate for localized esophageal cancer with stages T1, T2, or T3; N0; and M0 is 38%. For regional spread to nearby lymph nodes (N1, N2, or N3), the 5-year survival rate is 20%, and for distant lymph node or organ metastases (M1) it is 3% (American Cancer Society, 2013).
Diagnostic Studies
Laboratory abnormalities are often nonspecific in patients with esophageal cancer, but they may reflect the disease stage and assist clinicians in deciding on appropriate therapy. Bleeding may result in microcytic anemia. Malnutrition may reduce the serum albumin and cholesterol levels and the white blood cell count. Elevated liver enzyme levels may be an indication of hepatic metastases. Hypercalcemia, which has been associated with a poor prognosis, has been reported in patients with esophageal cancer (Basso, Roma, & Brunello, 2009). The role of serum tumor markers, such as carcinoembryonic antigen (CEA), cancer antigen (CA) 19-9, CA-50, and T-4, is uncertain. Although their levels are elevated in many patients, there is still no good evidence for their use in diagnosis or screening of esophageal cancer. Pulmonary function testing and arterial blood gas measurements are helpful to quantify the extent of chronic obstructive pulmonary disease. Patients with underlying coronary artery disease should have an echocardiogram to assess left ventricular function and a stress test to assess the extent of ischemic heart disease.
Presenting Signs and Symptoms of Esophageal Cancer |
Dysphagia Regurgitation Vomiting Weight loss Cough Pain Cachexia | Hoarseness Dyspnea Neck mass Hemoptysis Hematemesis Tracheoesophageal fistula Hiccups |
Source: Das (2010).
A plain chest x-ray provides limited information in patients with esophageal cancer. Possible abnormalities include an air–fluid level secondary to an obstructed esophagus, infiltrates suggesting aspiration pneumonia, tracheal deviation, pulmonary nodules, pleural effusion, and mediastinal widening from lymphadenopathy. The chest film, however, may be deceptively normal, even with advanced disease.
Regardless of how suspicious a lesion appears on contrast swallow, esophagoscopy with biopsy is mandatory to establish the diagnosis and is the gold standard (National Comprehensive Cancer Network [NCCN], 2013b). This is especially true if cancer is suspected and the barium esophagogram is normal. Flexible fiberoptic or video endoscopy permits the direct visualization of the esophageal tumor, its anatomic extent, and associated or secondary lesions.
Accurate tissue specimens can be obtained easily under direct visual control using endoscopic instruments. Multiple biopsy specimens provide a positive yield of 85% and in combination with brush cytology, accuracies of 90% to 100% should be readily attainable (American Cancer Society, 2013).
Upper endoscopy should also include direct visualization of the oropharynx, hypopharynx, epiglottis, vocal cords, and the stomach and duodenum. The ability to inspect these regions permits the detection of synchronous lesions and allows assessment of the suitability of the stomach and duodenum for esophageal replacement.
HER2 gene or protein testing of a tissue specimen can be done especially if esophageal cancer is too advanced or inoperable. The HER2 gene can determine if targeted therapy is applicable for treatment called Herceptin (trastuzumab).
CLINICAL STAGING
Once the diagnosis of esophageal cancer has been established histologically, staging of the tumor is the next critical step in determining which therapeutic option is appropriate.
CT of the chest and upper abdomen is now the standard noninvasive technique for staging esophageal cancer. It may be of value in determining surgical resectability, planning for radiation therapy, for CT-guided needle biopsies, or endoscopic palliation. CT scanning permits evaluation of esophageal wall thickness, mediastinal invasion, and the presence of regional or distant metastases. It is particularly useful in assessing local extension of disease and its relation to adjacent structures, especially when an oral contrast medium is used. It is less accurate in assessing the degree of periesophageal lymph node involvement and often underestimates the length of the esophageal lesion.
Experience with MRI seems to indicate that it does not have any particular advantage over CT and shares many of its limitations. A major problem with MRI is the lack of a suitable intraluminal contrast agent. MRI staging accuracy is about 40%, having low sensitivity and specificity. Another limitation of CT and MRI is that they lack the ability to differentiate layers of the esophageal mucosa (Puli et al., 2008). A positron emission tomography (PET) scan has value by providing assessment for cancer metastases.
Endoscopic ultrasound (EUS) can define the depth of tumor wall invasion and associated paraesophageal lymph nodes. The use of EUS may be limited if there is an obstructing tumor that cannot be traversed by the ultrasound probe. EUS has excellent sensitivity and specificity in accurately determining esophageal cancer staging even in an advanced stage. When EUS is used with a fine-needle biopsy, the sensitivity and specificity of both the EUS and the fine-needle biopsy are enhanced for evaluating esophageal cancers (Puli et al., 2008).
Depending on the location of the tumor, some patients may require additional staging modalities such as mediastinoscopy, bronchoscopy, laparoscopy, thoracotomy, or thoracoscopy. Bronchoscopy may be useful in patients with carcinomas of the upper and middle third of the esophagus to evaluate for airway invasion. Endoscopic evidence of invasion to the tracheobronchial tree precludes a safe esophagectomy. Similarly, laparoscopic and thoracoscopic staging of esophageal cancer has been found to be advantageous.
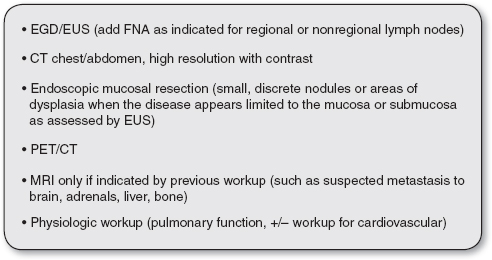
FIGURE 21.2
Current recommendations for workup for a newly diagnosed esophageal cancer.
EGD, esophagogastroduodenoscopy; EUS, endoscopic ultrasonography; FNA, fine-needle aspiration.
Source: Varghese et al. (2013).
See Figure 21.2 for current practice guidelines and recommendations for diagnosis and staging of esophageal cancer from the Society of Thoracic Surgeons (Varghese et al., 2013). These guidelines are also available on the Society’s website: www.sts.org/publications-resources/guidelines/diagnosis-and-staging-patients-esophageal-cancer
Treatment Options, Expected Outcomes, and Comprehensive Management
OPERATIVE MANAGEMENT
Surgical resection is the primary treatment modality for patients with cancer of the esophagus in the absence of known metastatic disease or medical contraindications to surgery. It is the only proven curative single-treatment modality for esophageal cancer and remains the gold standard by which all other therapeutic modalities are measured (NCCN, 2013b). Unfortunately, only half of patients have resectable disease at the time of presentation (Thallinger, Raderer, & Hejna, 2011). The goal of surgical resection is the eradication of all disease, including regional lymph nodes, while relieving dysphagia and maintaining GI continuity. Esophageal resection with lymphadenectomy offers the best chance for long-term survival in these patients; with many achieving 20% 5-year survival rates (American Cancer Society, 2013).
A variety of operative procedures are used for the resection of esophageal cancers and subsequent reconstruction of the alimentary tract. The choice of procedure depends on many factors, including the preference of the surgeon, the location of the tumor, the patient’s age and physiological fitness, and the extent of disease on EUS and intraoperative staging. Of these, location of the tumor and the surgeon’s preference are probably the two most important.
The most radical procedure is the en bloc resection. If preoperative staging is favorable, this procedure is undertaken with curative intent. The objective of en bloc resection is complete removal of the tumor with wide margins, together with most of the esophagus, adjacent connective tissue, and lymph nodes. Intraoperative radiation can be added as a salvage procedure to enhance patient outcomes (Jaroszewski & Winter, 2010). Reconstruction of the GI tract is usually accomplished with a gastric pull-up, but small bowel or colon can be substituted if necessary.
Regardless of the procedure used, surgical resection can be a formidable undertaking. Morbidity and mortality rates can be significant and are mainly caused by cardiopulmonary complications. A well-rehearsed interprofessional team of experienced surgeons, anesthesiologists, intensivists, and support staff is critical for a successful outcome.
RADIATION THERAPY
External beam radiation therapy as a single modality is considered palliative and results in few long-term survivors. Radiation therapy has not shown any benefit for improving local control or survival if it is dose escalated. The 5-year overall survival rates range from 0% to 21%, so radiation should not be used with curative intent (Shridhar et al., 2013). Radiation therapy is an important method of nonoperative palliation and provides relief from dysphagia in approximately 50% of patients, one half of whom will remain free of dysphagia until the time of death (Shridhar et al., 2013).
Endoluminal radiation therapy (brachytherapy) works by implanting a radioactive source directly at the tumor bed. Delivery of radiation by this means theoretically provides maximal effect to the tumor itself, with minimal risk to surrounding tissues. A complete or partial response has been seen in more than 70% of patients with significant relief of dysphagia and bleeding (Glynne-Jones & Harrison, 2013). Brachytherapy can provide better long-term palliation of dysphagia than metal stent placement (Shridhar et al., 2013). Unfortunately, nearly half of patients experience moderate to severe complications that require further therapy. Additional evaluation is therefore required to determine the ultimate role of this modality in the treatment of esophageal cancer.
CHEMOTHERAPY
Unlike surgery and radiation therapy, systemic chemotherapy has a theoretical advantage in treating esophageal cancer because most patients present with widespread systemic disease. Several drugs have been used against esophageal cancer, but when administered as single agents the responses are usually partial and short-lived. This has prompted an evaluation of combination chemotherapy; most protocols include cisplatin in combination with 5-fluorouracil (5-FU), bleomycin, methotrexate, and vindesine. Unfortunately, with the exception of the potential to improve resectability, none of these regimens has proven effective in providing local control or improving survival. These chemotherapeutic agents are currently utilized in combination with newer agents, such as paclitaxel, irinotecan, and oxaliplatin, or targeted biological agents in the reduction of locally advanced esophageal cancers and with distant metastases (van Hagen et al., 2012). The National Comprehensive Care Network (NCCN, 2013b) practice guidelines offer all current chemotherapy treatment modalities (www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf).
Therapies that target specific pathways activated in cancers can offer the potential for antineoplastic effects with minimal toxicities. These biological agents include epidermal growth factor receptor (EGFR) inhibitors, anti-angiogenic agents, cell cycle inhibitors, apoptosis promoters, and matrix metalloproteinase inhibitors. Concurrent biological targeted therapies are newer methods to treat esophageal cancers; combination therapy, including cetuximab and trastuzumab, may be a promising strategy to treat squamous cell carcinoma of the esophagus (Yamazaki et al., 2012).
MULTIMODAL THERAPY
Since the late 1970s, esophageal cancer trials have focused on adding chemotherapy to radiation therapy with surgical resection. The rationale is to control distant disease while dealing directly with the locoregional tumor. There is also the suggestion that certain chemotherapeutic agents may act as radiation sensitizers, thereby enhancing the efficacy of radiation therapy. Several ongoing Phase III trials are evaluating this form of therapy. Multimodal treatment using neoadjuvant chemoradiation followed by surgical resection is the standard of care, with current data demonstrating overall 3-year survival rates ranging from 30% to 60% (Merkow et al., 2012; Stahl et al., 2009; Tepper et al., 2008). Although surgical resectability has been improved, a clear survival advantage has not been seen. Most authors also report significant morbidity and mortality rates. The NCCN (2013b) offers guidelines for recommendations and combined treatment modalities for the various stages of esophageal cancers (www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf). Numerous clinical trials are ongoing for esophageal cancer patient enrollment (NCI, 2013a).
NONOPERATIVE OPTIONS
Because most patients with esophageal cancer present with advanced disease, curative treatment is generally not possible and palliation is the primary goal. Palliative options include surgery, radiation therapy, chemotherapy, endoscopic therapy, or supportive management only.
Mechanical dilatation is a simple and inexpensive modality that can afford relief of dysphagia in up to 70% of patients. Maloney (mercury-filled rubber) bougies, Eder-Puestow (metal olives), or Savary-Gilliard (tapered plastic) dilators have all been used successfully. Unfortunately, the procedure has a 15% failure rate and relief of dysphagia is short-lived. Complications, such as perforation and bleeding, occur in 1% of patients (Ho & Siddiqui, 2013; Ross & Kozarek, 2013). Dilatation is useful, however, as the initial step in several alternative nonoperative palliative techniques.
Transoral intubation with a prosthesis is the most popular worldwide method for palliating advanced esophageal cancer. Advantages include simplicity, short hospitalization, and immediate improvement in dysphagia. The overall reported mortality rate ranges from 3% to 15%, and a 20% to 60% complication rate has been reported (Witteman et al., 2009). Although most patients have improved swallowing, only 10% to 50% can eat solid food. The average length of survival after palliative intubation for esophageal cancer is <6 months (Witteman et al., 2009). Self-expanding metallic stents are used to improve the ease of insertion and to lessen the complications of the standard prosthesis (Gonda & Lightdale, 2012; Talreja et al., 2012).
Endoscopic laser therapy is most commonly performed with a Nd-Yag (neodymium–yttrium–aluminum–garnet) laser because of its deep tissue penetration and reliable hemostatic property. Relief of dysphagia occurs in 70% to 85% of patients, but multiple treatments are usually required. The dysphagia-free period is only 6 to 8 weeks.
Photodynamic therapy or PDT is a more selective form of laser treatment. This involves the use of special drugs, known as photosensitizing agents, and light to destroy cancer cells. This procedure requires an injection of a hematoporphyrin derivative agent that is selectively retained by neoplastic and reticuloendothelial tissues. Argon dye lasers or gold vapor lasers are typically used to activate the hematoporphyrin derivative, leading to release of free oxygen radicals and cell death. Photodynamic therapy is available and porfimer sodium (Photofrin) is approved by the Federal Food and Drug Administration (FDA) to relieve symptoms of esophageal cancer when it obstructs the esophagus or laser therapy alone cannot satisfactorily treat the cancer (Kim & Park, 2010). Although potentially curative for early tumors, there are possible complications, such as stricture, chest pain, nausea and vomiting, and a perforation rate of about 1% (Nava et al., 2011).
 GASTRIC CANCER
GASTRIC CANCER
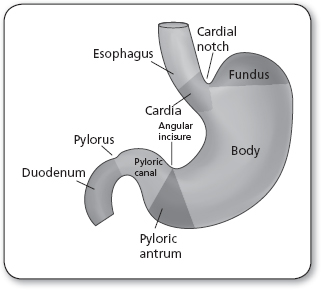
The stomach is a J-shaped, muscular, sac-like organ that lies between the esophagus and the small intestines (Figure 21.3). The stomach has five layers of mucosa, an esophageal and a pyloric sphincter, and is divided into sections known as the cardia, fundus, body, pyloric antrum, and pylorus. The stomach stores food and uses gastric juices to digest and break down food through peristaltic motion while passing it through to the small intestines for absorption (Ellis, 2011). Gastric cancer occurs when malignant cells form in the stomach lining, causing issues or interruption of this digestive process.
Cancer of the stomach is a disease with an almost uniformly poor prognosis. This, combined with its global distribution, is the second most common cause of cancer-related death in the world, making gastric cancer a major worldwide public health concern. Gastric cancer remains difficult to cure in Western countries, primarily because most patients present with advanced disease. Research into the basic biology, prevention, diagnosis, and treatment of this disease has accrued over the past century. In that time, though, the greatest reduction in overall morbidity and mortality rates has been realized through primary prevention via public health initiatives and tertiary treatment by the introduction and refinement of new surgical techniques.
Anatomy, Physiology, and Pathology
The vast majority of gastric malignancies are carcinomas. A carcinoma is a malignant tumor that comes from epithelial tissues. A carcinoma can invade or metastasize to nearby tissues, organs, lymph nodes, and various sites of the body (Carcinoma, n.d.). The incidence of gastric carcinoma increases with age. Carcinomas of the stomach have a long latency period that is often associated with recognizable precancerous lesions. Gastric acid inhibits the growth of bacteria within the stomach. Bacteria have been demonstrated to generate carcinogenic nitrosamine compounds from salivary and dietary nitrates. Conditions that lead to gastric achlorhydria may therefore predispose to gastric cancer. Intestinal metaplasia and chronic atrophic gastritis are two conditions associated with achlorhydria that have been linked with gastric cancer in correlation studies. Both chronic atrophic gastritis and intestinal metaplasia are more prevalent in areas with high rates of gastric cancer (Kim et al., 2010). Smoking, obesity, gastric ulcers, Epstein Barr virus, pernicious anemia, and prior radiation exposure are possible causes for the development of gastric cancers. About 10% of gastric cancers are familial (Dixon et al., 2013). The E-cadherin gene (CDH1) can be detected in 50% of diffuse-type gastric cancers (Guilford & Humar, 2013). Families that have this mutation have an autosomal dominant pattern of inheritance.
Helicobacter pylori is a microaerophilic, gram-negative, spiral-shaped bacterium that commonly has been found in patients with acute and chronic inflammation of the gastric antrum. Because of the known association between gastritis and gastric cancer, there has been speculation about the role of H. pylori in the causation of gastric cancer, and several large cohort studies have confirmed this suspicion. H. pylori infection is a major risk factor in about 80% or more of gastric cancers (Zhang et al., 2008). However, H. pylori infection is common in the general population, and most persons with H. pylori will not develop cancer (Suzuki, Iwasaki, & Hibi, 2009).
One reason for the lack of success in identifying a particular agent or sequence as the definitive cause of gastric cancer is the large number of strongly associated factors that have been studied. The sharp fall in gastric cancer in this country began in 1930, shortly after the passage and enforcement of the Pure Food and Drug Act of 1927. This realization contributed to a search for a nutritional cause of gastric cancer that began in the 1950s and continues today (Malekzadeh, Derakhshan, & Malekzadeh, 2009; Saghier, Kabanja, Afreen, & Sagar, 2013; Torres et al., 2013). It has been found that diets rich in preserved meats, smoked foods containing preformed N-nitroso compounds, and pickled foods containing nitrites, as well as diets low in fresh fruits and vegetables, are linked to a high risk of gastric cancer (Keszei, Goldbohm, Schouten, Jakszyn, & van den Brandt, 2013). High consumption of salted foods or alcohol is also closely correlated with an increased lifetime risk of developing gastric cancer (Bonequi, Meneses-González, Correa, Rabkin, & Camargo, 2013; Saghier et al., 2013). Conversely, diets high in raw fruits and vegetables and antioxidants such as vitamins C, E, and beta carotene are strongly correlated with a reduction in the risk of gastric cancer (American Cancer Society, 2013; Williams, 2013).
Two histologically distinct types of gastric carcinoma, intestinal and diffuse types, have been described (Sala et al., 2012). The distinction between intestinal and diffuse types of carcinoma continues to have relevance today, not only as a descriptive device but because the two types are thought to have different origins, risk factors, population distributions, and prognoses (see the “Epidemiology” section).
Intestinal-type gastric tumors are true adenocarcinomas. They are characterized by a proliferation of atypical glandular elements containing large numbers of mucin-containing goblet cells. There is commonly a loss of polarization with a varying amount of nuclear atypia in the cells lining these glandular formations. Histologically, the diffuse type of gastric carcinoma largely lacks recognizable glandular elements and consists predominately of stromal tissue. The hallmark of this type of tumor is the large number of signet-ring cells with deeply staining basophilic nuclei. Grossly, either of these tumors may be raised, flat, or ulcerated on endoscopic examination; in addition, intestinal-type tumors may be polypoid. The gross appearance of the lesion has little association with the true depth of invasion.
Noncarcinoma Gastric Malignancies
Although more than 90 of all gastric malignancies are adenocarcinomas, there are also three relatively uncommon tumors (Radulescu et al., 2012). The first of these is the primary gastric lymphoma. This represents 3% of all gastric malignancies and is predominately of the non-Hodgkin’s type (Conteduca et al., 2013). Gastric lymphomas arise from the mucosal-associated lymphoid tissue. The majority of these tumors is composed of B-lymphocytes and has long growth cycles, tending to remain within the stomach (Kelley & Duggan, 2003; Miyazaki, Takabe, & Yeudall, 2013). Primary T-cell lymphomas are much less common and tend to be more aggressive in their growth and spread. Although histiocytic lymphomas and true Hodgkin’s disease of the stomach have been reported in the literature, the incidence of these tumors is about 4% (American Cancer Society, 2013). Like lymphoma at most other sites in the body, gastric lymphomas tend to be highly responsive to radiation and chemotherapy. Nonetheless, surgical excision, where possible, remains an important part of the therapeutic regimen.
Prognosis for all of these tumors is related to the stage of the disease at diagnosis and the histological type of tumor. The 5-year survival rate for curative surgical resection ranges from 30% to 50% for patients with Stage II disease and from 10% to 25% for patients with Stage III disease (Jemal et al., 2011). Early B-cell lymphomas are reported to have a 75% 5-year survival rate and late-stage T-cell lymphomas as low as a 32% 5-year survival rate (Cai et al., 2012).
Gastric leiomyosarcomas constitute approximately 1% of all gastric malignancies (Insabato et al., 2012). These smooth muscle tumors can grow to a large size, protruding primarily into the peritoneal cavity rather than into the lumen of the stomach. They are usually slow-growing tumors and it can be challenging to distinguish them from benign leiomyomas. Often the definitive diagnosis of malignancy can be made only after surgical resection, when the entire specimen is available for examination. In most cases, though, size is a good indicator of malignancy: tumors <4 cm are likely to be benign, whereas those exceeding 6 cm are likely malignant (Stamatakos et al., 2009). Five-year survival rates after surgical excision have been reported to be as high as 55% for patients with smaller tumors but 30% for those with tumors >8 cm (Stamatakos et al., 2009).
The third major noncarcinoma tumor is the gastric carcinoid tumor. These endocrine tumors have been estimated to represent 1% of all gastric tumors (Nikou & Angelopoulos, 2012). Although histologically similar, the gastric carcinoids are divided into two classes based on the prevailing gastric mucosa (Matters, 2008; Nikou & Angelopoulos, 2012). The first class is the classic carcinoid tumor, which is strongly associated with chronic atrophic gastritis and Zollinger–Ellison syndrome. The hypersecretion of gastrin seen in these patients is thought to cause hyperplasia of enterochromaffin cells, resulting in carcinoid tumors. The second class of carcinoid-type tumors arises in histologically normal gastric mucosa without detectable antecedent endocrine abnormalities. These spontaneous or sporadic carcinoids are, in fact, true neuroendocrine carcinomas.
The gastritis-associated class of gastric carcinoid tumors is usually confined to the mucosa. Small tumors of this class are amenable to repeated endoscopic fulguration and treatment of the underlying gastric pathology, whereas larger tumors require surgical excision. The sporadic class is often poorly differentiated and more likely to metastasize to regional lymph nodes and distant sites. These tumors are rarely suitable for surgical intervention and have a markedly poor prognosis with a 5-year survival rate of 10% (Nikou & Angelopoulos, 2012).
Epidemiology
In the early part of this century, gastric cancer was the most common cancer in the United States. At that time, it accounted for 35% of cancers in men and 22% of cancers in women (exceeded only by uterine cancer). Beginning around 1930, and continuing until the middle of the last decade, the incidence of stomach cancer in the United States fell to its currently stable rate of 3 per 100,000 women and 6 per 100,000 men (American Cancer Society, 2013). In the United States, gastric malignancy is currently the 14th most common cancer. Gastric cancers occur at an older age, typically the seventh decade of life, in most of the population. Gastric cancer affects slightly more men than women (American Cancer Society, 2013). About 21,600 cases of stomach cancer will be diagnosed yearly. Men average approximately 13,230 cases whereas women average 8,370 cases. The number of people who will die of gastric cancer is approximately 10,990, or 6,740 men and 4,250 women. Asian and Pacific Islander males and females have the highest incidence of stomach cancer in the United States, followed by African American, Hispanic, White, and American Indian populations (American Cancer Society, 2013).
Worldwide, gastric cancer rates are about twice as high in men as in women. It is the fourth most common type of cancer. Stomach cancer is more common in Japan, China, Southern and Eastern Europe, and South and Central America. It is less common in Northern and Western Africa, South Central Asia, and North America (American Cancer Society, 2013).
The total incidence of gastric cancer has declined in Japan, Chile, and Venezuela because of the development of rigorous early screening that can detect cancer in the early stages. The decline in incidence has evolved in two apparently discrete populations of adenocarcinomas. The intestinal type (named for its histological appearance) appears to be responsible for the majority of the decline. This is also the type that seems to be affected by environmental and dietary factors, because the risk of this type is reduced if a person moves from an area of high prevalence to one of low prevalence (Leal et al., 2012). The other type of tumor is called diffuse because of its predilection toward widespread involvement of the stomach. There is evidence linking this type of gastric cancer to genetic factors because its incidence has not been found to be associated with specific environmental factors (Jemal et al., 2011). The incidence of diffuse-type gastric carcinoma has not changed significantly since its identification in 1965.
Diagnostic Criteria
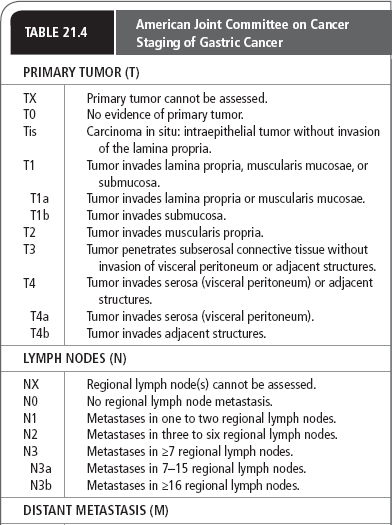
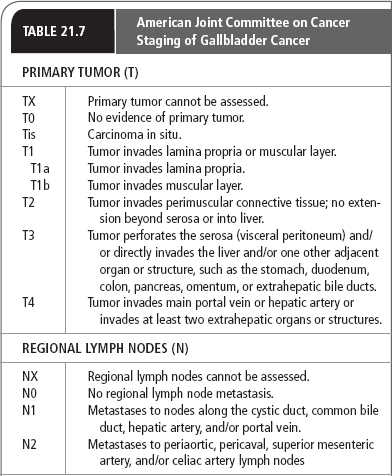
Several systems have been proposed to stage gastric carcinomas throughout the years. Currently, the system most commonly used by providers in the United States is the TNM system adopted by the AJCC (Table 21.4). This staging system is useful in standardizing communications about cancers by grouping them by extent and severity, which roughly correlates with prognosis.
The single most important property of the primary tumor in determining prognosis is the depth of invasion. The AJCC system classifies primary tumors as limited to the mucosa, extending to the serosa, or extending through the serosa (with or without involvement of contiguous structures). Regional lymph node involvement is divided into three groups corresponding to the number of regional lymph nodes found to be involved with tumor (see Table 21.4). Formerly classified as regional lymph nodes, the paraaortic, hepatoduodenal, pancreatic, and mesenteric lymph nodes are now classified as distant metastases.
History and Physical Examination
Gastric cancer is characteristically silent in its early stages. This contributes to the late stage at which most gastric cancers are diagnosed. Characteristic symptoms of gastric tumors such as indigestion, nausea or vomiting, dysphagia, loss of appetite, weight loss, early satiety, pain, anemia, obstruction, hematemesis, and melena, can appear late in the course of the disease. These symptoms herald extensive involvement of the gastric body or pylorus. Occasionally, large extraluminal tumors may be palpable through the abdominal wall. Linitis plastica (leather-bottle stomach) is a classic sign associated with tumor infiltration throughout the stomach. It manifests as extremely early satiety resulting from a dramatic decrease in gastric distensibility and can often be palpated through the abdominal wall in the right upper quadrant. Patients may exhibit signs such as pallor, a palpable enlarged stomach with a succussion splash, hepatomegaly, periumbilical metastasis, and enlarged lymph nodes.
DISEASE COURSE AND PROGNOSIS
Although advanced gastric cancer continues to have a uniformly poor prognosis, when gastric cancer is found and treated early, survival rates can be quite high. Approximately 1% to 3% of gastric cancers are associated with an inherited gastric cancer syndrome (NCCN, 2013c). The lifetime risk for hereditary diffuse gastric cancer (HDGC) in individuals with the gene mutation is approximately 67% for men and 83% for women. In patients with HDGC, gastric cancer occurs before the age of 40 years, with an average age at onset of 38 years. Germ-line mutations in the E-cadherin gene, CDH1, have been identified in families with HDGC and genetic testing is recommended. Management options for carriers of CDH1 germ-line mutations include prophylactic gastrectomy or intensive surveillance for early detection and treatment of gastric cancer (Fitzgerald et al., 2010).
The overall 5-year relative survival rate of people with stomach cancer in the United States is about 27% (American Cancer Society, 2013). Five-year survival rates with patients treated with surgery alone with Stages I, II, III, and IV disease are 71%, 46%, 20%, and 4%, respectively (American Cancer Society, 2013). The prognosis of patients with gastric cancer depends on the tumor extent, nodal involvement, and direct tumor extension beyond the gastric wall. Studies have looked at the fairly high rates of local regional recurrence after surgery, and the benefit of radiation therapy following surgery. One large intergroup study (0116 trial) randomized patients to surgery alone versus surgery with adjuvant chemoradiation. Radiation therapy consisted of 45 Gy given daily, and chemotherapy using an intravenous bolus of 5-FU and leucovorin given 4 weeks before radiation, during weeks 1 and 5 of radiation, and following radiation. Ten-year follow-up data demonstrated disease-free survival and overall survival favoring the chemoradiotherapy arm (Macdonald et al., 2009).
Patients with localized distal gastric carcinoma have a more than 50% cure rate, but these account for only 10% to 20% of all cases (Thun, DeLancey, Center, Jemal, & Ward, 2010). The 5-year survival rate for patients with proximal gastric cancer is only 10% to 15% (NCI, 2013b). Treatment for disseminated gastric cancer is to palliate symptoms, prolong survival, and improve quality of life (Shiraishi, Sato, Yasuda, Inomata, & Kitano, 2007).
Diagnostic Studies
The association between gastric cancer and chronic gastritis should alert providers to be on the lookout for neoplasia in patients with this condition. Laboratory testing for anemia can correlate with the patient’s clinical symptomatology such as fatigue and shortness of breath. Blood CEA is increased in 45% to 50% of gastric carcinoma cases and CA 19-9 is also elevated in about 20% (Kumar, Rajn et al., 2013). Routine upper GI endoscopy called an esophagogastroduodenoscopy (EGD) is the gold standard for the diagnosis, staging, and treatment of gastric cancer (Shah et al., 2013). EGD has a diagnostic accuracy of 95% and is the most common diagnostic test for initial evaluation (Mihaljevic, Friess, & Schuhmacher, 2012). Biopsies of suspicious areas have been shown to be of value in increasing early detection of gastric tumors. Upper GI series has an accuracy of 75% and can demonstrate the extent of disease when obstructive symptoms or bulky tumors are present thereby preventing an upper endoscope passage for examination of the stomach (Hoda, Rodriguez, & Faigel, 2009).
Practice guidelines from the NCCN (2013c; www.nccn.org/professionals/physician_gls/pdf/gastric.pdf) recommend the use of EUS, PET/CT, MRI, and laparoscopic techniques for clinical staging. The workup for newly identified gastric lesions should include CT or MRI. CT is the preferred mode of abdominal imaging and for preoperative staging. CT is relatively sensitive in detecting gastric wall infiltration, regional and distant lymph node abnormalities, and liver and spleen extension or metastases. CT has an accuracy between 43% and 82% for tumor staging (Pan et al., 2013). PET/CT has 68% accuracy for preoperative staging (Atay-Rosenthal, Wahl, & Fishman, 2012). MRI can also assess for a local disease process as well as evaluate potential areas of spread.
EUS probes can be applied to the mucosal surface of the stomach to determine the depth of tumor wall invasion or involvement of adjacent structures. In certain cases, even the regional lymph nodes can be examined with much higher accuracy than conventional transabdominal techniques. EUS is useful as a staging tool with up to a 95% accuracy when the CT scan fails to find evidence of T3, T4, or metastatic disease (Lei, Huang, Wang, Huang, & Huang, 2013). When the patient’s treatment modality will be neoadjuvant chemoradiotherapy for locally advanced disease, the health care provider depends on EUS data to improve patient stratification (Lutz et al., 2012). Laparoscopic staging can detect metastatic disease, with 31% accuracy for distant metastases in the periumbilical and hepatic lymph nodes (NCCN, 2013c).
Mass screening programs are used in countries that have populations at high risk of the development of gastric cancer. Such programs are performed in Japan, Chile, and Venezuela, where the incidence of gastric cancer is many times higher than in most places in the West. Screening is to detect gastric cancer in the early stages; it is controversial and not recommended in the United States because of the relatively low incidence. Methods and screening intervals vary in these countries and guidelines are continually updated. The only recommendation to screen for gastric cancer is the use photofluorography, the photography of x-ray images produced by a fluoroscopic examination (Jemal et al., 2011). Beneficial evidence is ongoing, with about 50% of the gastric cancers being detected in the early stage (Graham & Asaka, 2010; Hamashima et al., 2008; Verlato, Di Leo, Rossi, & de Manzoni, 2012).
Treatment Options, Expected Outcomes, and Comprehensive Management
Because gastric cancer is commonly diagnosed late in its course, often at a stage beyond which it can be successfully excised, and because of its historically poor response to chemotherapy and radiation treatment, multimodal treatment regimens are recommended (NCCN, 2013c). Curative therapy involves surgical resection, most commonly a total or subtotal gastrectomy, with an accompanying lymphadenectomy. Five-year survival rates for patients treated with surgical resection alone having Stage 0, I, II, III, and IV disease are 90%, 71%, 46%, 20%, and 4%, respectively (American Cancer Society, 2013).
SURGICAL TREATMENT
The first-line treatment of newly diagnosed gastric cancer continues to be surgical excision. In most cases, total gastrectomy with attempts to remove all involved tissues back to microscopically clean margins is the ultimate goal. Approximately 90% of patients treated by gastrectomy with lymphadenectomy will have a survival rate >5 years (Lee, Kim, et al., 2012). Such an operation is currently not possible for the majority of patients presenting with locally advanced or metastatic cancers (Lee et al., 2009). In the United States, it is recommended that patients receive a gastrectomy, with adjacent lymph node removal, known as a D1 lymphadenectomy, with a goal to remove at least 15 lymph nodes (Sano, 2013).
The standard surgical treatment in Japan has differed from that in Western countries. Japanese surgeons are generally more aggressive and have had very high success rates by doing a more extensive removal of the lymph nodes surrounding the cancer, known as a D2 lymphadenectomy. The results of such surgery have yielded significant improvements in long-term survival rates in Japanese populations, with reports of the 5-year survival rates for 2008 being 92% for Stage I and 16% for Stage IV cancers (Isobe et al., 2011). Attempts to reproduce these results in Europe and the United States have been successful (Hanna, Boshier, Knaggs, Goldin, & Sasako, 2012). Recent studies demonstrate that extended lymphadenectomy in gastroesophageal cancer resection is safe for Western patients and survival rates can be improved to achieve those comparable to rates seen in Japan (de Manzoni, Di Leo, & Verlato, 2012; Hanna et al., 2012; Kodera, 2013).
The radical nature of surgical gastrectomy for gastric cancer (whether Japanese or Western) is fraught with intra- and postoperative dangers. These are often long procedures associated with massive intra- and extracellular fluid shifts. Patients are frequently malnourished and compromised by pulmonary or cardiac abnormalities. In addition, many of these operations involve removal of the pylorus or pancreas, leaving the patient prone to dumping syndrome or postoperative diabetes (Kodera, 2013). Clearly, such treatment requires detailed preoperative discussion to ensure that the patient understands both the nature of the disease process and the risks of the surgical resection.
RADIOCHEMOTHERAPEUTICS
Chemotherapy is used alone in patients with advanced gastric cancer to help relieve signs and symptoms. The use of chemotherapy in conjunction with surgery and adjuvant combination chemotherapy has shown some efficacy after curative resection. A systematic review of 22 randomized controlled trials, 13 meta-analyses, and two secondary analyses formulated a clinical practice guideline with several recommendations (Knight et al., 2013). Postoperative 5-FU-based chemoradiotherapy based on a Macdonald approach or perioperative ECF (epirubicin, cisplatin, fluorouracil) chemotherapy based on the Cunningham/MAGIC (Medical Research Council Adjuvant Gastric Infusional Chemotherapy) approach was recommended for overall survival in patients with resectable gastric cancer. The overall survival was significantly improved with the use of either postoperative chemoradiation (Macdonald approach) or perioperative ECF (MAGIC protocol), with both being acceptable standards of care in North America (Knight et al., 2013).
These agents affect all rapidly proliferating cell populations. This includes bone marrow suppression, with its immunocompromising effect; GI mucosal sloughing, with concomitant malabsorption; and impairments in wound healing and regeneration of skin appendages, resulting in, among other things, hair loss. Nausea and vomiting, anorexia, diarrhea, stomatitis, neutropenia, thrombocytopenia, peripheral neuropathy, fatigue, and shortness of breath are other common side effects.
Another strategy is chemotherapy given before surgery, known as neoadjuvant chemotherapy, which can shrink the gastric cancer for ease of resection (Liao, Yang, Peng, Xiang, & Wang, 2013). Downstaging of tumors preoperatively may make curative resection more feasible; however, a survival benefit was not demonstrated in this study.
Finally, true multimodal therapy for gastric cancer is the standard of care (Knight et al., 2013). This approach makes use of neoadjuvant chemotherapy to shrink the tumor, followed by resection and lymphadenectomy with postoperative chemoradiotherapy. In patients with node-positive (T1 N1) and muscle-invasive (T2 N0) disease, postoperative chemo-radiation therapy should be considered. Adjuvant external-beam radiation therapy with combined chemotherapy has been evaluated in the United States (Chakravarthy et al., 2012; Sherman et al., 2013). Clinical trials are ongoing and patients can contact the NCI for clinical trial enrollment (Bang et al., 2012).
Palliation with several combined chemotherapy agent groups (fluorouracil [5-FU], epirubicin, cisplatin [ECF]; epirubicin, oxaliplatin, and capecitabine [EOX]; cisplatin and 5-FU [CF]; docetaxel, cisplatin, and 5-FU; etoposide, leucovorin, and 5-FU [ELF]; 5-FU, doxorubicin, and methotrexate [FAMTX]) are recommended for Stage IV gastric carcinoma and have demonstrated a survival benefit (Lei et al., 2012).
Other biological therapies such as trastuzumab, cisplatin, and either 5-FU or capecitabine can be used for patients with HER2-positive tumors. Targeted therapy uses drugs that attack specific abnormalities within cancer cells. Targeted therapy tends to have fewer severe side effects than standard chemotherapy agents (Smyth & Cunningham, 2012). Endoluminal laser therapy, endoluminal stent placement, or gastrojejunostomy can alleviate gastric obstruction. Additionally, radiation therapy can palliate bleeding, pain, and obstruction.
Current clinical practice guidelines for the diagnosis, treatment, and survival of gastric cancer are available from the NCCN (2013c; www.nccn.org/professionals/physician_gls/pdf/gastric.pdf).
 GALLBLADDER CANCER
GALLBLADDER CANCER
Even though there have been many advances in the diagnosis and management of GI cancer, carcinoma of the gallbladder remains a rare and challenging tumor with a poor overall prognosis. Many of these tumors are not resectable at the time of presentation. Common symptoms that are caused by gallbladder cancer are jaundice, pain, and fever. The 5-year survival rate is <5% in most reported series (Eggert et al., 2013). This dismal image, however, has been changing. Advances in hepatobiliary imaging have led to earlier diagnosis and a better understanding of the pathology of gallbladder cancer, and aggressive surgical intervention has led to improved survival for many patients.
Anatomy, Physiology, and Pathology
The gallbladder is a distensible storage reservoir that allows bile acids to be delivered in a high concentration and controlled manner to the duodenum for the solubilization of dietary lipids. It is a pear-shaped organ 5 to 12 cm long located in a fossa on the undersurface of the liver in a plane dividing the liver into right and left lobes (Figure 21.4). It is held to the liver by a peritoneal covering and anatomically is composed of four parts: fundus, corpus, infundibulum, and neck. The fundus is the widest part of the gland and often reaches to the free anterior edge of the liver, where it might contact the anterior abdominal wall near the lateral border of the right rectus muscle. The neck leads to the cystic duct, and when distended by a stone has the appearance of a diverticulum known as Hartmann’s pouch. The cystic duct runs from the neck of the gallbladder to the junction of the common hepatic duct and common bile duct.
The gallbladder is lined by a mucosa of columnar epithelium, and the wall consists of mucosa, lamina propria, tunica muscularis, and serosa. Macroscopically, gallbladder cancer may appear as a nodule, a polypoid mass, or a focal or diffuse thickening of the gallbladder wall that may infiltrate the surrounding structures.
Histologically, more than 90% of gallbladder cancers are adenocarcinoma (Goldin & Roa, 2009). Other histological types include papillary adenocarcinomas, which make up 6% of all types of gallbladder cancer (these tend to be well differentiated and carry a more favorable prognosis); undifferentiated or anaplastic carcinoma; squamous cell carcinoma; and adenocanthoma or mixed adenosquamous tumors (Dutta, 2012). In general, papillary tumors are superficial lesions projecting into the lumen and are less likely to invade the liver or spread to regional lymph nodes (Dutta, 2012). In contrast, nodular tumors are more likely to infiltrate early, invade the liver, and metastasize to lymph nodes (Dutta, 2012). Other rare tumors of the gallbladder include carcinoid, melanoma, lymphosarcoma, reticulum cell sarcoma, fibrosarcoma, rhabdomyosarcoma, leiomyosarcoma, and pleomorphic cell sarcoma.
Epidemiology
INCIDENCE
Carcinoma of the gallbladder is the most common biliary tract malignancy and the fifth most common GI cancer (Srivastava, Srivastava, Sharma, & Mittal, 2011). In the United States, 10,310 new cases and 3,230 deaths from gallbladder cancer were estimated in 2013. (American Cancer Society, 2013). Gallbladder carcinoma is an uncommon and highly fatal malignancy. The SEER database places the incidence of 1 to 2 cases per 100,000 population in the United States. The rate for men in comparison to women is 1,260 men to 1,970 women (Randi et al., 2009; Wang et al., 2008). The frequency increases with age, an important risk factor, and reaches a peak during the seventh and eighth decades of life, with the average age of diagnosis being above 73 years. Seventy-five percent of gallbladder carcinoma cases occur in people older than 65 years. Obesity can increase the risk, as can certain cysts and abnormalities of the bile ducts (Lazcano-Ponce et al., 2001).
People from the Andean area, North American Indians, and Mexican Americans have the highest incidence rate of gallbladder cancer. In Europe the highest rates are found in Poland, the Czech Republic, and Slovakia. Incidence rates in other regions of the world are relatively low. The highest mortality rates for gallbladder cancer are in South America, 3.5 to 15.5 per 100,000 among Chilean Mapuche Indians, Bolivians, and Chilean Hispanics. Intermediate rates, 3.7 to 9.1 per 100,000, are from Peru, Ecuador, Colombia, and Brazil. Lower mortality rates are observed in North America, with the exception of higher rates in New Mexico American Indians (11.3 per 100,000) and Mexican Americans (Lazcano-Ponce et al., 2001).
ETIOLOGY
Although the exact etiology remains unclear, several factors are known to increase the risk of gallbladder cancer (Table 21.5). Of these, gallstones are the most common, mainly because of the high prevalence in the general population. This association has been noted for many years, and most series report that more than 90% of patients with gallbladder cancer have coexistent chronic cholecystitis and cholelithiasis (McKnight & Patel, 2012). Despite this, there is no conclusive evidence that this is a cause-and-effect relationship. In fact, only 1% of patients requiring a cholecystectomy for cholelithiasis have an unsuspected gallbladder cancer (Clemente et al., 2012). Prophylactic cholecystectomy, therefore, cannot be recommended for patients with asymptomatic gallstones, perhaps with one exception: patients with a calcified or “porcelain” gallbladder have a 12% to 60% risk of cancer and should be advised to undergo elective cholecystectomy (Furlan, Ferris, Hosseinzadeh, & Borhani, 2008).
Studies have suggested that patients with an anomalous pancreaticobiliary duct junction, with or without a choledochal cyst, are at risk of gallbladder cancer (Lee et al., 2011). In addition, biliary excretion is a common mode of clearance of toxic metabolites. As a result, various carcinogens have been suspected of causing biliary tract neoplasms. Although no gallbladder carcinogen has yet been identified in humans, animal studies suggest that methylcholanthrene, azotoluene, and nitrosamines can cause gallbladder cancer (Singh, Ansari, & Narayan, 2012). Rubber-plant workers also appear to be at increased risk, as are those in the automobile, wood-finishing, and metal-fabricating industries (Boutros, Gary, Baldwin, & Somasundar, 2012).
Biliary tract malignancy has also been found to be more prevalent in patients with ulcerative colitis. Although the majority involve the bile ducts, as many as 12% originate in the gallbladder (Noda, Chiba, Toyama, & Konishi, 2009). Typhoid carriers appear to be at increased risk of all types of hepatobiliary carcinoma. Epidemiological studies have suggested a strong relationship between gallbladder cancer and obesity and estrogens (Stinton & Shaffer, 2012; Zhang et al., 2013); however, both of these factors are also associated with an increased incidence of gallstones. Finally, evidence suggests that gallbladder adenomas, especially polyps larger than 1 cm, have a malignant potential (Lee, Yun, et al., 2012; Miller & Jarnagin, 2008; Park, Kim, Choi, & Lee, 2010). In these patients, elective cholecystectomy is recommended.
Risk Factors for Gallbladder Cancer |
Cholelithiasis
Chronic cholecystitis
Calcified “porcelain” gallbladder
Choledochal cysts
Anomalous pancreatobiliary duct junction
Ulcerative colitis
Typhoid carriers
Obesity
Estrogens
Gallbladder polyps
Rubber plant, automobile, wood-finishing, and metal-fabricating work
Carcinogens (methylcholanthrene, azotoluene, nitrosamines)
Source: Stinton and Shaffer (2012).
Adjacent organ invasion is most commonly on the liver and usually presents at diagnosis as a biliary obstruction. Periportal and peripancreatic lymphadenopathy, hematogenous metastases, and peritoneal metastases are other areas of spread. The anatomic position of the gallbladder, and the vagueness and nonspecificity of symptoms, contribute to the late diagnosis. The poor prognosis is related to the advanced stage at diagnosis.
Diagnostic Criteria
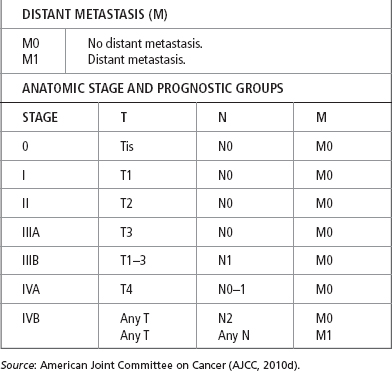
There is no uniform staging classification for carcinoma of the gallbladder. Earlier studies used the Nevin system, which is based on the depth of invasion and the spread of tumor (Nevin, Moran, Kay, & King, 1976). A number of modifications of this staging system have been used, and it has been shown to have prognostic significance. Table 21.6 shows the 5-year survival rates by TNM staging. More recently, the TNM classification, as outlined by the American Joint Committee on Cancer (AJCC, 2010d; Table 21.7), has found wide acceptance, although alternative systems are still in use in Europe and Japan. In addition to depth of invasion and local spread, histological grade (based on degree of differentiation) has been found to be an important determinant of survival. Virtually all long-term survivors of gallbladder cancer have well-differentiated tumors. No classification system, however, correlates all aspects of gross and histological pathology into an ideal staging system (Marsh et al., 2012).
History and Physical Examination
Five-Year Survival Rates by TNM Classification |
STAGE | 5-YEAR SURVIVAL RATE (%) |
0 | 80 |
I | 50 |
II | 28 |
IIIA | 8 |
IIIB | 7 |
IVA | 4 |
IVB | 2 |
TNM, Tumor–Node–Metastasis system.
Source: Data from American Cancer Society (2013).
Most patients present with a variety of nonspecific digestive complaints. The most common symptom is abdominal pain, and more than half of the patients present with either biliary pain or acute cholecystitis. Nausea, vomiting, weight loss, jaundice, fever, severe itching, and black tarry stools are other common symptoms. The majority of patients had symptoms for 6 months or less before presentation. A right upper quadrant mass may be apparent and is usually tender. The jaundice is accompanied by pain in the majority of cases. The presence of a palpable gallbladder represents advanced disease, as do malaise, anorexia, fever, and weight loss. Jaundice is most often caused by invasion of the common bile duct or compression from involved pericholedochal lymph nodes and, less frequently, involvement of the liver.
DISEASE COURSE AND PROGNOSIS
The 5-year survival rate of gallbladder cancer patients diagnosed at or before Stage I is more than 50% (American Cancer Society, 2013). As with most intra-abdominal cancers, the pattern of spread may be hematogenous, lymphatic, direct extension, or peritoneal seeding. Spread to adjacent organs is most common and usually involves the liver and other surrounding structures such as the duodenum, colon, and anterior abdominal wall. The common hepatic duct is frequently involved by direct extension, particularly with tumors originating in the neck of the gallbladder or Hartmann’s pouch. In this way, gallbladder cancer can closely mimic the clinical and radiological features of hilar bile duct tumors (Khan et al., 2012). Lymphatic spread occurs early and follows the lymphatics to the lymph nodes around the cystic duct, common hepatic duct, common bile duct, and pancreatic duodenal region. Between 25% and 75% of patients have lymph node metastases at the time of presentation (Khan et al., 2012). Distant hematogenous and intraperitoneal spread is rare and is usually seen only in the late stages of disease.
The majority of patients with carcinoma of the gallbladder have advanced unresectable disease at the time of presentation. As a result, fewer than 5% of all patients are alive after 5 years. However, results are much better when survival is evaluated according to tumor stage (Boutros et al., 2012; see Tables 21.6 and 21.7).
Diagnostic Studies
Because of the nonspecific clinical features, preoperative diagnosis is relatively rare, and most patients present with advanced unresectable disease. With the exception of jaundice, no specific physical findings or laboratory abnormalities are present. Blood tests for bilirubin, albumin, alkaline phosphatase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transpeptidase (GGT) can be abnormally high and are so in liver or gallbladder disease.
Tumor markers such as CEA and CA 19-9 are high especially in advanced disease. CA 242 is particularly useful in differentiating malignant from benign biliary disorders with a specificity of 84%; CA125 and alpha-fetoprotein (AFP) are nonspecific and have not been helpful in diagnosis (Dutta, 2012). However, with the use of imaging techniques, combined with a high index of suspicion, preoperative diagnosis can be achieved in many patients. Ultrasonography, CT, MRI, cholangiography, and angiography have all been used to evaluate gallbladder carcinoma.
High-resolution ultrasonography (HRUS) has increased the accuracy and sensitivity in detecting gallbladder cancer and may detect the disease at an early and potentially curable stage (Joo et al., 2013; Miller & Jarnagin, 2008). It detects features associated with gallbladder cancer, such as stones, liver metastases, retroperitoneal node enlargement, and dilated bile ducts. Ultrasonography can also show specific anatomical features such as a complex mass filling the gallbladder lumen, marked wall thickening, a polypoid or fungating mass, and direct invasion of the liver, which may suggest the correct diagnosis. In several series, ultrasonography has shown great diagnostic accuracy (Joo et al., 2013; Konstantinidis et al., 2012; Miller & Jarnagin, 2008), and it should be routinely performed in the investigation of gallbladder disease when carcinoma is suspected. EUS can provide detailed images of the gallbladder because the gallbladder has close proximity to the duodenum. EUS has been helpful in evaluating penetration, staging, and extension of regional disease (Kim et al., 2012). EUS has 100% accuracy in staging gallbladder tumors in situ (Tis): 76% for T1, 85% for T2, and 93% for T3–4 (Dutta, 2012).
CT can also be used to assess gallbladder abnormalities. It may demonstrate an intra-abdominal mass, a mass replacing the gallbladder, or a mass extending outside of it. Spiral CT can demonstrate invasion of the adjacent liver, bile duct wall thickness, and hilar vessels, which is important for staging (Dutta, 2012; Valls, Ruiz, Martinez, & Leiva, 2013).
MRI can also be used to diagnose gallbladder cancer. MR cholangiography and vascular enhancement techniques can be used to assess the resectability of invasive cancers (Dutta, 2012; Valls et al., 2013).
Selective angiography and CT portography have been reported to have high diagnostic accuracy and may provide information regarding tumor resectability (Singh & Facciuto, 2012). Local extension may be visualized and hepatic arterial or portal vein encasement may be confirmed. This information, however, may be provided by spiral CT and MRI without the need for an invasive technique.
Intravenous cholangiography and oral cholecystography have a very limited place in the assessment of hepatobiliary disease. Percutaneous transhepatic cholangiography (PTC) and endoscopic retrograde cholangiopancreatography (ERCP), however, may be of value in detecting gallbladder cancer (Dutta, 2012; Valls et al., 2013). Biliary ductal anatomy can be visualized and operative procedures planned accordingly.
If radiological studies suggest that a gallbladder cancer is present and surgically resectable, preoperative establishment of a tissue diagnosis is not required. However, if resection is not possible, tumor biopsy will be needed to plan nonoperative methods of palliation. Cytological examination of bile or of brushings of obstructed ducts has a low yield. Ultrasound- or CT-guided biopsy will usually be successful, and laparoscopic biopsy has been found helpful in establishing a diagnosis and confirming the stage of disease (Miller & Jarnagin, 2008). Laparoscopic cholecystectomy should not, however, be used as a means of removing a gallbladder with cancer.
Magnetic resonance cholangiopancreatography (MRCP) is a noninvasive test to image the bile ducts. ERCP is an invasive procedure and biopsies can be obtained in addition to placing a stent into the biliary duct to keep it patent. A PTC is used if an ERCP cannot be performed.
Treatment Options, Expected Outcomes, and Comprehensive Management
SURGICAL RESECTION
The most effective therapy for cancer of the gallbladder is surgical resection of the primary tumor and areas of local extension (Dutta, 2012). Unfortunately, fewer than 25% of patients have resectable lesions (D’Hondt et al., 2013). Palliative surgery is used when the tumor is unresectable, to relieve pain, correct an obstruction of the bile ducts, or prevent complications.
If the disease is limited to the mucosa, cholecystectomy is adequate therapy, and survival rates approach 100% (Yi et al., 2013). This stage of disease is most often found incidentally at the time of cholecystectomy for benign biliary disease. If the cancer is at a very early stage (T1a) and is completely removed, then no further therapy is recommended, as long as the cystic duct margin is not involved and the disease is limited to the mucosa (Dutta, 2012).
For more invasive lesions, therapy is less well defined. Tumors infiltrating beyond the mucosa require more than simple cholecystectomy. A radical or extended cholecystectomy has become standard in many centers. This procedure includes segmental resection of the liver with regional lymphadenectomy. Patients with disease limited to the mucosa, muscularis, or subserosa can achieve 5-year survival rates of 50% to 80% (American Cancer Society, 2013; Dutta, 2012). Even with serosal involvement, extended cholecystectomy provides a survival advantage of approximately 30% over simple cholecystectomy without increased mortality (Dutta, 2012). This operation should be the procedure of choice for all preoperatively recognized and resectable gallbladder cancers.
When tumor extends beyond the confines of the gallbladder, it remains unclear how extensive a resection should be performed. There have been a few reports of long-term survivors after hepatic resection or hepatopancreaticoduodenectomy for advanced gallbladder cancer. The morbidity and mortality rates are high, and these procedures are indicated only in a very select group of patients (Sakamoto et al., 2013).
PALLIATION
Although radical surgery appears an attractive proposition for localized disease, most patients present with advanced disease that cannot be resected with clear margins. Palliation thus becomes the goal of therapy, with the administration of adjuvant chemotherapy in all patients with advanced gallbladder cancer (Dutta, 2012). A variety of chemotherapeutic agents have been used for the treatment of gallbladder cancer. Common side effects with patients receiving chemotherapy for gallbladder cancer are hair loss, stomatitis, anorexia, nausea, vomiting, diarrhea, neutropenia, thrombocytopenia, and fatigue.
Radiation therapy has been used in patients with both resected and unresectable disease. Although external beam radiotherapy may help to reduce pain or relieve biliary obstruction, no survival advantage has been reported. Intraoperative radiotherapy and/or brachytherapy in combination with resection or external beam radiotherapy have been used as adjuvant therapy.
Radiation therapy in combination with chemotherapeutic radiosensitizing agents can also be used to treat advanced disease, especially if the cancer does not respond to standard chemotherapy agents. Chemoradiation is a combination of radiation therapy with chemotherapy agents such as 5-FU or capecitabine that can make the radiation more effective. These two chemotherapeutic agents are given with radiation therapy and are better tolerated with combined modalities. One study found that combining gemcitabine and cisplatin may help people live longer than getting gemcitabine alone. A multi-institutional, randomized, Phase III study (NCT01149122) of advanced biliary cancers evaluated the benefit of chemotherapy (gemcitabine and oxaliplatin) in combination or without erlotinib but did not meet the end point for improvement in overall survival and progression-free survival (Lee, Park, et al., 2012). For advanced disease, chemotherapy improves quality of life and survival, and gemcitabine with cisplatin represents the standard of care (Caldow Pilgrim, Groeschl, Quebbeman, & Gamblin, 2013). Numerous clinical trials continue to evaluate the effects of immunogenic chemotherapeutic agents on survival (Vacchelli et al., 2013).
Many patients present with biliary obstruction caused by extrahepatic duct compression from direct extension of tumor or by lymph node metastases. If unresectable disease is found at the time of surgery, a biliary bypass procedure can produce effective palliation. A nonoperative approach, with either transhepatic or endoscopic stenting, can relieve jaundice and may be the preferred choice when there are excessive operative risks.
Current clinical practice guidelines for diagnosis, treatment, and survival of gallbladder cancer are available from the NCCN (2013d; www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf).
 PRIMARY LIVER CANCER
PRIMARY LIVER CANCER

Full access? Get Clinical Tree