FIG. 14.1 Components of the capillary pressure gradient. Filtration reflects the difference between the combined forces that push fluid out of the capillary (capillary pressure and interstitial fluid colloid osmotic pressure) and those that attempt to hold fluid in the capillary (plasma colloid osmotic pressure and interstitial fluid pressure). (From Copstead LE, Banasik JL: Pathophysiology, ed 5, St. Louis, MO, 2013, Elsevier.)
Electrolytes
Electrolytes are any substance in solution that contains free ions that make the substance electrically conductive (e.g., elements, chemicals, minerals). These ions carry an electric charge. The cations are positively charged and include sodium, potassium, calcium, and magnesium. The anions are negatively charged ions and include chloride, bicarbonate, phosphate, sulfate, and ions of inorganic acids such as lactate. Protein also carries a negative charge at physiologic pH. Each of the fluid compartments of the body contains electrolytes. The concentration and specific composition of electrolytes in each compartment vary, and the number of cations in each compartment balances the number of anions to maintain electric neutrality.
The major ions found in the ECF are sodium and chloride. Potassium and phosphate are predominantly intracellular ions. The predominance of sodium outside the cell and potassium inside the cell is the result of a cell membrane pump that exchanges sodium and potassium ions. This active transport mechanism requires energy from adenosine triphosphate. Although electrolytes constitute only a small fraction of the body weight, they are essential for facilitation of normal body function. They maintain electroneutrality and chemical conditions in the body fluids, equilibrium between ECF and ICF, and regulation of neuromuscular activity. Laboratory testing of electrolyte concentrations is usually done before and after surgery, many times requiring that the blood is drawn in the PACU.
Sodium
Sodium is the major cation in the ECF. Blood plasma sodium averages 135 to 145 mEq/L and usually does not vary (±5 mEq/L). Variations greater than this can affect many physiologic activities; therefore, mechanisms for regulation of sodium concentration are of prime importance in maintenance of balance. Basically, the body regulates sodium with conservation mechanisms when the sodium is low. If body stores of sodium are high, the body excretes sodium primarily via the kidneys and, to a lesser extent, through sweat and feces.
The body fluids are maintained in an isotonic state with regulation of the concentration of sodium and its most abundant anion, chloride. Concentrations of sodium and chloride in the fluids are maintained primarily via loss or retention of water. Loss of salt is accompanied by loss of water and retention of salt by retention of water. Water moves into areas where salt is in higher concentration, which is why patients are often placed on a low-salt diet in an effort to reduce fluid overload on the heart and other major organs. However, patients who receive magnesium sulfate can also have impaired fluid excretion.
Of particular interest to the perianesthesia nurse are patients who have undergone urologic surgery and have been or are currently receiving irrigation fluids in the bladder. These patients are at risk of developing hyponatremia. The most common surgical procedure associated with this complication is a transurethral resection of the prostate. Irrigation fluids typically consist of sorbitol and mannitol or glycine in distilled water. The irrigants are isotonic. The amount of irrigation solution absorbed through the venous sinuses in the bladder averages 10 to 30 mL per minute of resection time. For this reason, the resection time should ideally be limited to 1 hour or less. The absorption of the irrigating fluid results in the fluid entering the vascular system; this can lead to volume overload and ultimately to dilutional hyponatremia.
The resulting lowered serum sodium concentrations can cause serious cardiac and neurologic consequences. Concentrations of sodium at 140 mEq/L are usually associated with the development of cardiac dysrhythmias that can lead to cardiac arrest. Progressive neurologic symptoms include restlessness, confusion, nausea, vomiting, coma, and convulsions.
Hypernatremia is most often caused by a loss of body fluids resulting in excess sodium. Elective surgery should be postponed until sodium levels greater than 150 mEq/L are corrected. Correction of water deficit should take place over 48 hours with hypotonic solutions. Rapid correction can result in seizures, cerebral edema, and coma.3
Potassium
Potassium is the most important intracellular cation. Measurement of intracellular potassium is difficult; therefore, only extracellular potassium is measured. The normal values are between 3.5 and 5.5 mEq/L. Potassium affects the excitability of nerve and muscle tissue and is important in the maintenance of cardiac rhythm, deposition of glycogen in liver cells, and transmission and conduction of nerve impulses. It also contributes to cellular energy production. Overall, abnormal potassium concentrations can have serious effects on the contractility of the heart, resulting in dysrhythmias and potential cardiac arrest.
Potassium depletion can be accompanied by changes in plasma potassium concentration. True depletion develops only with a net loss of potassium, whereas a decrease in plasma potassium, hypokalemia, can occur with a shift of potassium from the ECF to the ICF. Decreased intake can cause a mild deficit because the mechanisms for potassium conservation are not as efficient as those for sodium. Severe depletion results from abnormal losses rather than decreased intake. The most common causes of severe potassium loss are usually associated with diuretics (see Chapter 13), vomiting, acute blood loss, gastrointestinal surgery, and nasogastric suctioning. Cardiac arrhythmias, polyuria, confusion, and weakness of skeletal muscle are commonly observed in mild hypokalemia. Hallucinations, diminished reflexes, ST-segment depression, widened QRS, flattened T waves, and cardiac arrest could result from severe depletion. Oral replacement with potassium chloride is in the range of 60 to 80 mEq/day. Peripheral intravenous (IV) potassium should not exceed 8 mEq/h so as not to irritate veins. Central IV potassium can be infused at 10 to 20 mEq/h. The administration of 0.5 mEq/kg of potassium chloride usually raises the serum potassium concentration by 0.6 mEq/L. If the patient is receiving catecholamine drugs, the increase is approximately 0.1 mEq/L; if the patient is receiving beta-adrenergic antagonists, the serum concentration increases by approximately 0.9 mEq/L. It is important to note that correction of hypomagnesemia may be needed to avoid the increased loss of potassium by the kidneys.
Hyperkalemia is often associated with situations in which cells are injured or destroyed. Examples include chronic and acute renal failure, crush injuries, burn victims, and newborns who receive relatively large transfusions. The administration of succinylcholine, a depolarizing skeletal muscle relaxant, can also produce hyperkalemia (discussed in Chapter 23). Accidental lethal doses of supplemental IV potassium have been administered to patients with rapid intravenous infusion in the PACU. Cases have been recorded in which death occurred within 5 minutes of the rapid injection of just 25 mEq of potassium; therefore, under no circumstance should potassium chloride be given via IV push.3
Calcium
Calcium is one of the major extracellular cations. It is deposited in the bone tissue as crystalline salts composed primarily of calcium and phosphate; the remainder is in the plasma, ISF, and soft tissues. The major fraction of calcium that accounts for its physiologic effects is the ionizable calcium in plasma, of which the normal plasma concentration is maintained between 4.0 and 5.6 mg/dL. The remainder is bound to protein and other substances in nonionizable form with normal serum calcium levels rending 8.5 to 10.5 mg/dL. Calcium has an important function in neuromuscular transmission, skeletal muscle contraction, blood coagulation, and exocytosis necessary for the release of neurotransmitters and autacoids (e.g., serotonin, histamine, kinins). In addition, the balance of the appropriate calcium concentration is controlled by the parathyroid hormone, calcitonin, and vitamin D. Calcium also has a reciprocal relationship with phosphate ions.
Hypocalcemia can result from any number of causes such as hypoparathyroidism, pancreatitis, renal failure, or decreased serum albumin levels. In hypocalcemia, the nervous system becomes progressively more irritable as the membrane becomes increasingly permeable to sodium. At a certain critical level of calcium, the nerve fibers become so irritable that they begin to fire spontaneously. Impulses pass to skeletal muscles and can cause skeletal muscle spasm including laryngospasm. Severe tetanic spasms are called tetany. Hypocalcemia occurs when the serum calcium concentration is lower than approximately 8 mg/dL. Neuromuscular function becomes increasingly impaired with decreased myocardial contractility, increased central venous pressure, and hypotension. Because of skeletal muscle spasm and potential laryngospasm, when caring for patients with hypocalcemia, the perianesthesia nurse should have appropriate airway equipment readily available for resuscitation (Fig. 14.2).4
An increased secretion of parathyroid hormone, most commonly caused by a parathyroid tumor, can cause hypercalcemia. In this situation, nervous system depression results in reduced reflex activity, and depression of muscle contractility results in skeletal muscle weakness, constipation, and loss of appetite. Because some calcium is excreted in the urine, a mild hypercalcemia can induce kidney stones as the calcium combines with phosphate or other anions and precipitates.
Phosphorus
Phosphorus is a major intracellular anion; most of it (85%) is located in the bones. It probably represents the single most important mineral constituent in cellular activity. Normal serum laboratory values range from 3.4 to 4.5 mg/dL for adults and fluctuates with age; it is higher in children. Phosphorus serves many functions including forming red blood cells (RBCs) and acting as an intermediary in the metabolism of carbohydrates, proteins, and fats (glycolysis). Phosphorus promotes deposition of calcium in the bone and is essential for the delivery of oxygen via the RBCs to body tissues. The small number of phosphorus ions in plasma is important in acid-base balance by way of the phosphate buffer system. Phosphorus is also important in regulation of energy metabolism in the form of adenosine triphosphate. It is also active in the functions of DNA.
Magnesium
Magnesium is an essential element found primarily in muscle and bone. It effects tissue irritability and is a cofactor in various enzyme reactions. Magnesium has a significant effect on cardiac cell membrane ion transport and is essential for activation of many enzyme systems. Magnesium is an essential regulator of calcium within cells and is the natural physiologic antagonist of calcium. In regard to skeletal muscle contraction, the presynaptic release of acetylcholine depends on the actions of magnesium. The current reference values for magnesium range from 1.6 to 2.4 mEq/L.
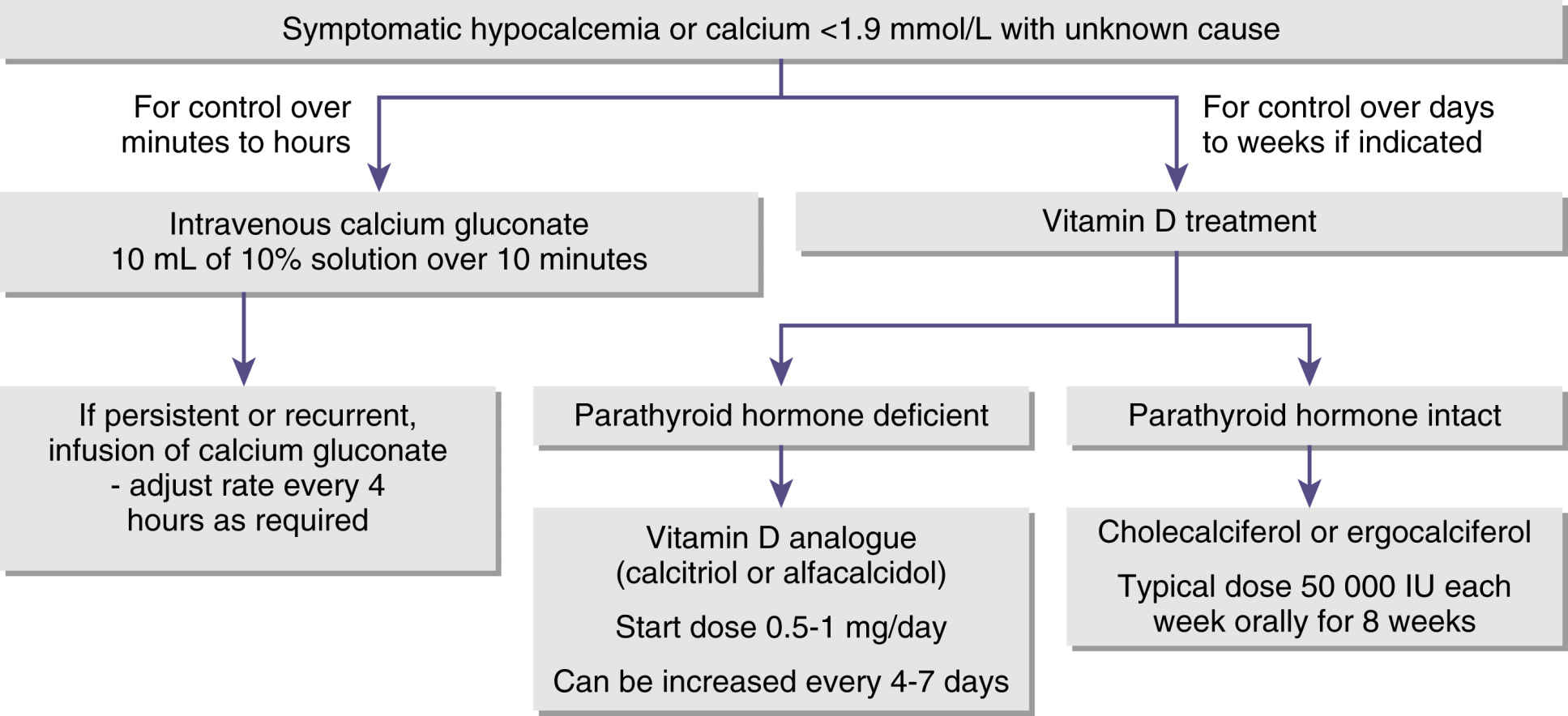
FIG. 14.2 Algorithm for managing hypocalcemia. (From Cooper MS, Gittos NJ: Diagnosis and management of hypocalcemia. Br J Med 336(7656):1298–1302, 2008.)
Hypomagnesemia is frequently overlooked as an electrolyte deficiency. Patients with alcoholism, poor diets or starvation, total parenteral nutrition without supplementation, nasogastric suctioning, or protracted vomiting or diarrhea can have this syndrome. Patients who have undergone cardiopulmonary bypass surgery are susceptible because of the dilutional effects of the pump-priming solutions. Symptoms of acute hypomagnesemia can include Chvostek and Trousseau signs as with hypocalcemia, stridor, skeletal muscle weakness, seizures, and coma. In the perianesthesia period, ventricular dysrhythmias are usually the most common symptom of hypomagnesemia. Treatment for this syndrome is magnesium 1 to 2 g IV over 15 to 60 minutes or a continuous infusion of magnesium at 0.5 to 1.0 g/h. With severe life-threatening hypomagnesemia, an infusion of magnesium of 10 to 20 mg/kg is usually administered over 10 to 20 minutes.
Hypermagnesemia is a rare clinical phenomenon. The most common cause of hypermagnesemia is the parenteral administration of magnesium as a treatment for pregnancy-induced hypertension. Symptoms of hypermagnesemia include sedation, myocardial depression, relaxed skeletal muscles and, when severe, paralysis of the muscles of ventilation. Treatment of the life-threatening hypermagnesemia is with calcium gluconate (1 g) given intravenously followed by a loop diuretic and increased fluid loading to produce diuresis in an effort to enhance the excretion of excess magnesium. Monitoring for vasodilation and negative inotropic effects is critical.2
Perioperative Blood and Fluid Replacement
Because of many factors (e.g., NPO, insensible fluid loss, surgical stresses of hemostatic function), fluid status, medical and surgical history, and medication regimens should be assessed. If problems with hemostasis are envisioned, coagulation function should be assessed before surgery to ensure appropriate intraoperative and postoperative coagulation. A patient can lose up to 75% of RBC volume if the total blood volume is maintained with the administration of colloid or crystalloid solutions. If the red cells are not replaced, the result is a loss in oxygen-carrying capacity because RBCs carry approximately 90% of the oxygen in the blood. In the situation of massive transfusions, many complications can arise in the PACU such as dilutional coagulopathy, acidosis, electrolyte abnormalities, and other long-term consequences as described in Chapter 29.

Full access? Get Clinical Tree








