Chapter 85 Fluid and electrolyte therapy
The management of patients’ fluid and electrolyte status requires an understanding of body fluid compartments as well as an understanding of water and electrolyte metabolism. These principles will be considered along with the commonly encountered fluid and electrolyte disturbances. Recent evidence for the use of fluid therapy in a number of common clinical scenarios will also be presented.
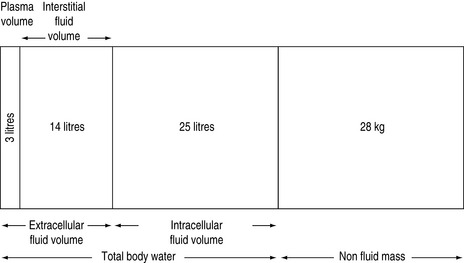
FLUID COMPARTMENTS (Table 85.1, Figure 85.1)
TOTAL BODY WATER
In humans, water contributes approximately 60% of body weight, with organs varying in water content (Table 85.2). The variation of the percentage of total body weight as water, between individuals, is largely governed by the amount of adipose tissue. The average water content as a percentage of total body weight is 60% for males and 50% for females. Total body water as a percentage of total body weight decreases with age, due to a progressive loss of muscle mass, causing bone and connective tissue to assume a greater percentage of total body weight1–3 (Table 85.3).
Table 85.1 Body fluid compartments
| Fluid compartment | Volume (ml/kg) | % Total body weight |
|---|---|---|
| Plasma volume | 45 | 4.5 |
| Blood volume | 75 | 7.5 |
| Interstitial volume | 200 | 20 |
| Extracellular fluid volume | 250 | 25 |
| Intracellular fluid volume | 350 | 35 |
| Total body fluid volume | 600 | 60 |
Table 85.2 Water content of various tissues
| Tissue | % Water content |
|---|---|
| Brain | 84 |
| Kidney | 83 |
| Skeletal muscle | 76 |
| Skin | 72 |
| Liver | 68 |
| Bone | 22 |
| Adipose tissue | 10 |
Table 85.3 Water content as a percentage of total body weight
| Age (years) | Males (%) | Females (%) |
|---|---|---|
| 10–15 | 60 | 57 |
| 15–40 | 60 | 50 |
| 40–60 | 55 | 47 |
| > 60 | 50 | 45 |
Total body water is commonly divided into two volumes, the extracellular fluid (ECF) volume and the intracellular fluid (ICF) volume.2 Sodium balance regulates the ECF volume, whereas water balance regulates the ICF volume. Sodium excretion is normally regulated by various hormonal and physical ECF volume sensors, whereas water balance is normally regulated by hypothalamic osmolar sensors.4
WATER METABOLISM
Water balance is maintained by altering the intake and excretion of water. Intake is controlled by thirst, whereas excretion is controlled by the renal action of antidiuretic hormone (ADH). In health, plasma osmolalities of about 280 mOsm/kg suppress plasma ADH to concentrations low enough to permit maximum urinary dilution.5 Above this value, an increase in ECF tonicity of about 1–2% or a decrease in total body water of 1–2 l, causes the posterior pituitary to release ADH, which acts upon the distal nephron to increase water reabsorption. Maximum plasma ADH concentrations are reached at an osmolality of 295 mOsm/kg.5 The osmotic stimulation also changes thirst sensation and, in the conscious ambulant individual, initiates water repletion (drinking), which is more important in preventing dehydration than ADH secretion and action. Thus, in health, the upper limit of the body osmolality (and therefore serum sodium) is determined by the osmotic threshold for thirst, whereas the lower limit is determined by the osmotic threshold for ADH release.6
Increase in osmolality caused by permeant solutes (e.g. urea) does not stimulate ADH release. ADH may also be released in response to hypovolaemia and hypotension, via stimulation of low and high pressure baroreceptors. ADH release is extremely marked when more than 30% of the intravascular volume is lost. ADH may also be stimulated by pain and nausea, which are thought to act through the baroreceptor pathways.4 ADH release may also be stimulated by a variety of pharmacological agents (Table 85.4). Renal response to ADH depends upon an intact distal nephron and collecting duct, and a hypertonic medullary interstitium. The capacity to conserve or excrete water also depends upon the osmolar load presented to the distal nephron.4
Table 85.4 Drugs affecting ADH secretion
| Stimulate | Inhibit |
|---|---|
| Nicotine | Ethanol |
| Narcotics | Narcotic antagonists |
| Vincristine | Phenytoin |
| Barbiturates | |
| Cyclophosphamide | |
| Chlorpropamide | |
| Clofibrate | |
| Carbamazepine | |
| Amitriptylline |
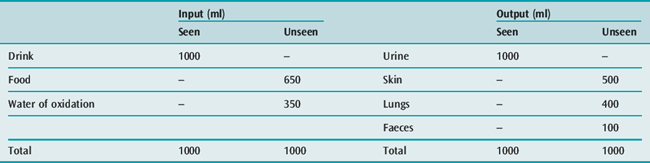
WATER REQUIREMENTS
Water is needed to eliminate the daily solute load, and to replace daily insensible fluid loss (Table 85.5). With a normal daily excretion of 600 mOsm solute, maximal and minimal secretions of ADH will cause urine osmolality to vary from 1200 to 30 mOsm/kg respectively, and the urine output to vary from 500 ml to 20 l/day respectively. Skin and lung water losses vary, and may range from 500 ml to 8 l/day depending on physical activity, ambient temperature and humidity.
DISORDERS OF OSMOLALITY
TONICITY
Osmolality is a measure of the number of osmol/kg water. The osmolality of the ECF is due largely to sodium salts. Clinical effects of hyperosmolality, due to excess solute, depend upon whether the solute distributes evenly throughout the total body water (e.g. permeant solutes of alcohol or urea) or distributes in the ECF only (e.g. impermeant solutes of mannitol or glucose). With impermeant solutes, hyperosmolality is associated with a shift of fluid from the ICF to the ECF compartment. Hyperosmolality due to increased impermeant solutes is knownas hypertonicity. This condition may also be associated with a reduction in the serum sodium concentration (see below).
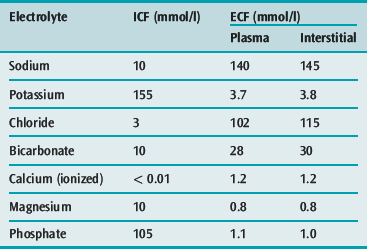
ELECTROLYTES
Chemical compounds in solution may either:
SODIUM
Sodium is the principal cation of the ECF and accounts for 86% of the ECF osmolality. In a 70-kg man, total body sodium content is 4000 mmol (58 mmol/kg) and is divided into a number of compartments (Table 85.7). ECF concentration of sodium varies between 134 and 146 mmol/l. The intracellular sodium concentration varies between different tissues, and ranges from 3 to 20 mmol/l.
Table 85.7 The sodium compartments in a 70-kg man
| Total (mmol) | (mmol/kg) | |
|---|---|---|
| Total body sodium | 4000 | 58 |
| Non-exchangeable bone sodium | 1200 | 17 |
| Exchangeable sodium | 2800 | 40 |
| Intracellular sodium | 250 | 3 |
| Extracellular sodium | 2400 | 35 |
| Exchangeable bone sodium | 150 | 2 |
The standard Western society dietary sodium intake is about 150 mmol/day, but the daily intake of sodium varies widely, with urinary losses ranging from < 1 to > 240 mmol/day.7 Sodium balance is influenced by renal hormonal and ECF physical characteristics. The complete renal adjustment to an altered sodium load usually requires 3–4 days before balance is restored.
HYPONATRAEMIA
Hyponatraemia is defined as a serum sodium less than 135 mmol/l and may be classified as isotonic, hypertonic or hypotonic, depending upon the measured serum osmolality (Table 85.8).
Table 85.8 Common causes of hyponatraemia
Isotonic hyponatraemia
Plasma normally contains 93% water and 7% solids (5.5% proteins, 1% salts and 0.5% lipids). If the solid phase is elevated significantly (e.g. in hyperlipidaemia or hyperproteinaemia), any device which dilutes a specific amount of plasma for analysis will give falsely lower values for all measured compounds. This effect produces ‘factitious hyponatraemia’ and is associated with a normal measured serum osmolality.8 Measurement of plasma sodium by an ion-selective electrode is not affected by the volume of plasma ‘solids’ and therefore ‘pseudohyponatraemia’ will not occur with this method.8
Hypertonic hyponatraemia
In patients who have hypertonicity due to increased amounts of impermeant solutes (e.g. glucose, mannitol, glycerol or sorbitol), a shift of water from the ICF to the ECF occurs to provide osmotic equilibration, thus diluting the ECF sodium. Such resultant hyponatraemia is often associated with an increased measured osmolality. For example, in the presence of hyperglycaemia, for every 3 mmol/l rise in glucose, the serum sodium decreases by 1 mmol/l.9
Hypotonic hyponatraemia
Hyponatraemia is almost always caused by an excess of total body water, due to excessive hypotonic or water-generating i.v. fluids (e.g. 1.5% glycine, 0.45% saline or 5% glucose) or excessive ingestion of water, particularly in the presence of high circulating ADH concentrations. It may rarely be caused by loss of exchangeable sodium or potassium. In the latter circumstances, a loss of approximately 40 mmol of sodium or potassium, without a change in total body water content, is required to lower the serum sodium by 1 mmol/l. As hyponatraemia may be associated with an alteration in both total body water and total body sodium, the ECF may be increased (hypervolaemia), decreased (hypovolaemia) or exhibit no change (isovolaemia).5
Transurethral resection of prostate (TURP) syndrome
Clinical features
The TURP syndrome consists of hyponatraemia, cardiovascular disturbances (hypertension, hypotension, bradycardia), an altered state of consciousness (agitation, confusion, nausea, vomiting, myoclonic and generalised seizures) and, when using glycine solutions, transient visual disturbances of blurred vision, blindness and fixed dilated pupils, following TURP. It has also been described following endometrial ablation.10 It may occurwithin 15 minutes or be delayed for up to 24 hours postoperatively,11 and is usually caused by an excess absorption of the irrigating fluid which contains 1.5% glycine with an osmolality of 200 mosm/kg. Hyponatraemic syndromes have also been described when irrigating solutions containing 3% mannitol or 3% sorbitol have been used. Symptomatology usually occurs when > 1 l of 1.5% glycine or > 2–3 l of 3% mannitol or sorbitol are absorbed.12
The excess absorption of irrigating fluid causes an increase in total body water (which is often associated with only a small decrease in plasma osmolality), hyponatraemia (as glycine, sorbitol or mannitol reduces the sodium component of ECF osmolality) and an increase in the osmolar gap.12,13 When glycine is used, other features include hyperglycinaemia (up to 20 mmol/l; normal plasma glycine concentrations range from 0.15 to 0.3 mmol/l), hyperserinaemia (as serine is a major metabolite of glycine), hyperammonaemia (following deamination of glycine and serine), metabolic acidosis and hypocalcaemia (due to the glycine metabolites glyoxylic acid and oxalate). Because glycine is an inhibitory neurotransmitter, and as it passes freely into the intracellular compartment when glycine solutions are used, hyperglycinaemia may be more important in the pathophysiology of this disorder than a reduction in body fluid osmolality and cerebral oedema.14
Treatment
Treatment is largely supportive with the management of any reduction in plasma osmolality being based on the measured plasma osmolality and not the plasma sodium. If the measured osmolality is > 260 mOsm/kg and mild neurological abnormalities exist, and if the patient is haemodynamically stable with normal renal function, close observation and reassurance (e.g. the visual disturbances are reversible and will last for less than 24 hours) are usually all that is needed. If the patient is hypotensive and bradycardic with severe and unresolving neurological abnormalities, haemodialysis may be warranted. Hypertonic saline is only used if the measured osmolality is < 260 mOsm/kg and severe non-visual neurological abnormalities exist.15
Syndrome of inappropriate ADH secretion (SIADH)
This syndrome is a form of hyponatraemia in which there is an increased concentration of ADH inappropriate to any osmotic or volume stimuli that normally affect ADH secretion.16 Diagnostic criteria and common causes are listed in Tables 85.9 and 85.10, respectively.
Table 85.9 Criteria for the diagnosis of syndrome of inappropriate antidiuretic hormone (SIADH)
Table 85.10 Aetiologies of syndrome of inappropriate antidiuretic hormone (SIADH)