Chapter 19 Fiberoptic and Flexible Endoscopic-Aided Techniques
I. Introduction and Historical Background
II. Flexible Fiberoptic and Non-fiberoptic Bronchoscope Design and Care
III. Rationale for Fiberoptic Intubation
V. Clinical Techniques of Fiberoptic Intubation
VII. Special Flexible Fiberoptic Bronchoscope Uses
VIII. Rarely Recommended: Oxygen Insufflation via Bronchoscope
IX. Fiberoptic Intubation in Infants and Children
X. Advantages of Flexible Fiberoptic Bronchoscopes
XI. Disadvantages and Complications of Flexible Fiberoptic Bronchoscopes
XII. Other Causes of Failure with Fiberoptic Bronchoscopes
XIII. Learning Fiberoptic Intubation
XV. Introducing Fiberoptic Intubation into One’s Own Practice
I Introduction and Historical Background
The purpose of this chapter is threefold:
1. Incorporate recent airway management developments for integration with flexible endoscopes.
2. Impart knowledge concerning flexible endoscopic techniques and tips for airway use.
3. Increase the likelihood that readers might achieve a level of expertise in flexible endoscopic intubation and airway management.
Historically, a wide variety of devices have been employed to visualize the airway in living human subjects. Manuel Garcia, a singing instructor, ingeniously used mirrors to reflect sunlight in 1854, allowing him to examine his own moving vocal cords. He was considered the “first laryngoscopist” after a presentation to the Royal Society of Medicine.1 Using a blind digital technique, William Macewen completed the first endotracheal intubation under anesthesia as well as the first awake intubation for surgery and anesthesia in 1878.2 On hearing of an accidental intratracheal insertion in 1895, Alfred Kirstein used an esophagoscope that incorporated a proximal light source and spatula-like area to perform the first “direct” laryngoscopy.3
Bronchoscopy history began to develop in 1887, when Gustav Killian extracted a foreign body from a farmer’s respiratory tract with a rigid bronchoscope. Chevalier Jackson modified this rigid bronchoscope by inclusion of a distal tungsten light bulb and suction channel in 1904, resulting in the significantly greater likelihood of successful endotracheal intubation.4
Finally, a remarkable development took place in 1966. Shigeto Ikeda proposed his ideas for construction of a flexible fiberoptic bronchoscope (FOB) to two companies, Machida Endoscope and Olympus.5 Meanwhile, in 1967, Peter Murphy ingeniously used a choledochoscope for endotracheal intubation.6 In 1968, the Machida company at last produced an extremely “bendable,” guided FOB, which featured an eyepiece for seeing images transmitted along 15,000 glass fibers that were 14 µm in diameter. Clinically, Ikeda’s technique was to insert this very flexible instrument through a rigid bronchoscope. Later that year, Olympus produced a more maneuverable FOB with a working channel that allowed the application of cytology brushes. Ikeda’s new FOB pictures of the distal airway, presented at the International Congress on Disease of the Chest in Copenhagen, were instantaneously news-making revelations. He was able to demonstrate a stand-alone FOB insertion method for endotracheal intubation in many countries, and he published his experiences in 1971.7
Initially many clinicians advanced FOB use for diagnostic purposes, for ordinary intubation procedures, and, eventually, for airway management in patients with very challenging airways.8–12 The inherent characteristic of FOB superiority forged its involvement as an instrument of first choice in difficult intubation (DI) cases, cervical spine risk scenarios, and as a diagnostic and therapeutic tool for patients with hypoxemia, high airway pressure, pulmonary tumors, infection, foreign bodies, airway stenosis, obstructive sleep apnea, tracheomalacia, and many more wide-ranging abnormalities.
Raj advocated the role of FOB assistance in double-lumen tube (DLT) placement11 while Ovassapian further delineated its advantages in one-lung isolation in 1987.13 Through FOB use, Benumof compared bronchial insertions of right and left DLTs to explain why the relationship of bronchial anatomy to DLT construction inevitably resulted in less successful right-sided DLT positioning.14
In the 1980s, the Asahi Pentax Company’s integration of the FOB with a preexisting invention, the charge-coupled device (CCD), permitted video monitor viewing of the airway and heightened its educational and clinical benefits.15 FOB construction creativity has continued to evolve, with sizes available for all types of patients, many featuring full-color imaging and a working suction/injection channel. Light-emitting diodes (LED) and micro video complementary metal-oxide semiconductor chips (CMOS) for transmission capability have also been implemented.
Although universal acceptance of FOB use for intubation was somewhat slow for many years, Ovassapian and colleagues were leading and effective pioneers in the educational promotion of its use through workshop training and simulation, eventually popularizing this astonishingly effective technique.16 Currently, the FOB resides worldwide within all algorithms for DI, and there is a clear, growing tendency to use this technique in combination with other recent advances as a multimodal approach to difficult airway (DA) management.
II Flexible Fiberoptic and Non-Fiberoptic Bronchoscope Design and Care
A Fiberoptic Bronchoscope Design
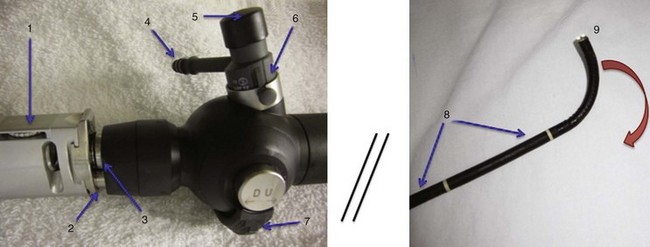
Variations in design may seem endless, but all flexible endoscopes have certain commonalities. Differences are described later in this chapter. There are three main parts: handle, insertion tube, and flexible tip (Fig. 19-1).
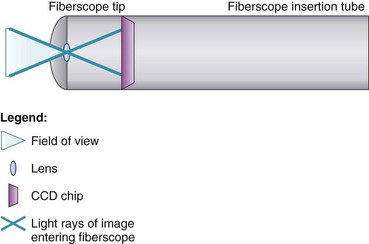
At the end of the handle is either a battery-operated light source, which allows for more portability, or an optical cable connection to an external light source. The proximal “visual section” of the handle is the location of an eyepiece and lens, a video output adaptor, or an actual video screen with an integrated camera (depending on the model). FOBs with a CCD or CMOS camera chip at the tip have a wide-angle image and a higher resolution of the picture transmitted, compared with older models, which have a narrower field of view and less optical detail (Fig. 19-2). This makes video fiberscope deployment useful clinically, particularly in teaching.
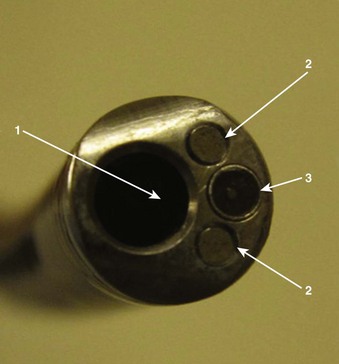
Four specific components travel inside the insertion tube along the FOB length (Fig. 19-3). The first allows the transmission of light going toward the tip by way of one or two light guide bundles constructed of noncoherent glass fibers. A high degree of light intensity is focused on the proximal light guide bundle, but heat filters or reflecting mirrors prevent injury to the insertion tube components.
On the back of the handle is a bending or angulation lever. Through an up or down movement of the thumb, it causes pulling on the third insertion tube component located along the sagittal plane, the two angulation wires. This initiates movement of the FOB tip in the opposite direction (i.e., down or up, respectively) by as much as 240 to 350 degrees (see Fig. 19-1). These delicate wires can also be broken with excessive pressure, including lever motion if the FOB tip is still within the ETT lumen.
The FOB tip or bending section is hinged and has an objective lens (approximately 2 mm in diameter) with a fixed focal point and a short field of view (75 to 120 degrees) (see Figs. 19-1 to 19-3).
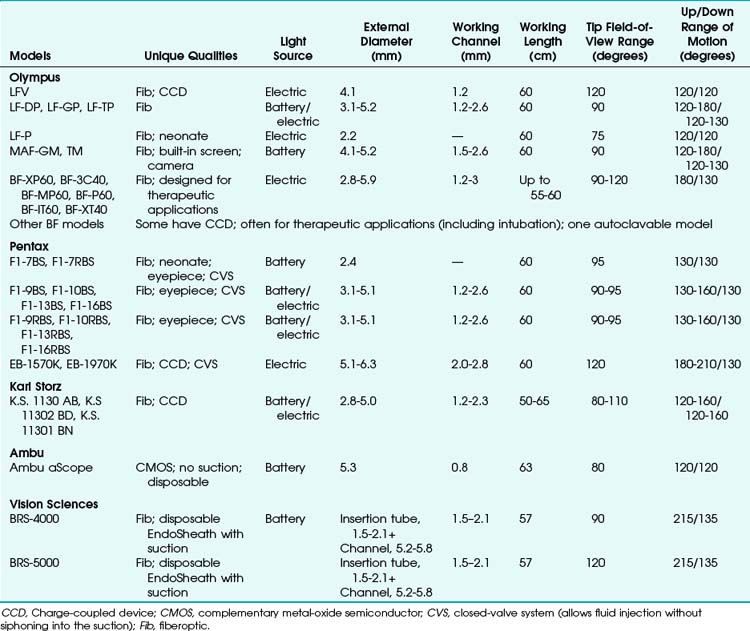
Many models are available in sizes from premature infant to large adult and with varying fields of view, video monitors, closed-valve systems, and other features (Table 19-1).
B Fiberoptic Bronchoscope Cleaning
Almost half a million bronchoscopies are performed per year in the United States.17 Whenever any equipment is reused between patients, there is always a concern for avoiding the transmission of communicable diseases. Although there have not been any reports of infection or cross-contamination caused by fiberoptic intubation for elective or emergency purposes, sporadic references testify to instances of infection or contamination after flexible bronchoscopic procedures such as bronchial washings or bronchoscopic lavage. Between the years 2000 and 2007, the American Association for Respiratory Care (AARC), American College of Chest Physicians (AACP), American Association for Bronchology and Interventional Pulmonology (AABIP), and Association for Professionals in Infection Control (APIC) issued guidelines and consensus statements to assist in the prevention of FOB-associated infection or contamination.18–20
Since the 1970s, sources of contamination have included a number of factors: sentinel patients, contaminated water, inadequate sterilization technique (due to insufficient quality or quantity of sterilizing solution), repeated use of cleaning fluid or brushes, automated endoscope reprocessors, and FOB instruments with design errors or damage.21–24 Valves and working channels are usually the most suspect areas for ineffective FOB sterilization. Multiple organisms have been found, including Pseudomonas aeruginosa, non-tuberculosis mycobacteria, Serratia marcescens, Mycobacterium tuberculosis, Stenotrophomonas maltophilia, Legionella pneumophila, Rhodotorula rubra, Klebsiella species, Proteus species, and fungi.21,25–28
Disinfection may take up to 45 min (Fig. 19-4). The assistant should carefully insert a cleaning brush into portals entering the working channel. The brush must be advanced fully along the compete span of this conduit to effect early removal of secretions according to the instructions of the manufacturer and the health care organization.
The suction ports and biopsy ports (if present) need to be disassembled from the rest of the FOB. All nondisposable parts must be gently placed in glutaraldehyde, peracetic acid, orthophthaldehyde, hydrogen peroxide gas plasma, or other manufacturer-indicated sterilization solution, and the working channel must be syringe-flushed with this disinfectant conforming to manufacturer and health care organization instructions.29–31
After completion of the specified sterilization time, all parts must be removed to prevent caustic damage to the instrument. The FOB should be gently washed and thoroughly rinsed with sterile water, including flushing of the working channel to prevent toxicity from residual chemicals.32 It is mandatory to dry the channel by suctioning or purging with 70% alcohol and compressed air for a time period in keeping with manufacturer and health care organization instructions.
Most bacteria, fungi, and viruses are susceptible to these sterilization processes, including human immunodeficiency virus (HIV) and hepatitis. FOBs that have been used in patients with contagious diseases (e.g., tuberculosis) may require a lengthy ethylene oxide sterilization and aeration procedure for up to 24 hours, with the venting cap secured to the venting connector. In the case of suspected prion infection (Creutzfeldt-Jakob or variants), it is best to avoid bronchoscopy if possible, because the sterilization process for most medical instruments would be damaging.18–20,33
Presently, routine surveillance cultures have not been recommended by the AARC, AACP, AABIP, or APIC because of lack of criteria to determine testing frequency, relevance when positive results occur, indeterminate courses of action, and costs of testing procedures.18–20
During all handling and sterilization techniques, keeping the FOB as straight as possible is extremely important to avoid damage. Once the device has been cleaned, it is preferable to store it suspended by its handle in a moderately lighted, climate- and humidity-controlled, safe location. Close proximity to radiation should be avoided because of deleterious effects on the FOB material.34
C Disposable Flexible Bronchoscopes
1 Sheathed Fiberoptic Bronchoscope
Vision Sciences, Inc. (Orangeburg, NY) has produced two adult-sized, channel-less FOBs for use with a clear, durable, snugly fitting, presterilized flexible, thermoplastic, elastomer EndoSheath that incorporates its own 1.5- to 2.1-mm working channel. The disposable sheath potentially prevents transmission of organisms and may increase FOB availability by requiring no down-time for sterilization. It might also reduce costs in centers where economic factors limit the purchase of more than a minimum number of bronchoscopy systems or limit the availability of sterilization equipment or personnel.35,36
2 Non-fiberoptic Flexible Bronchoscope
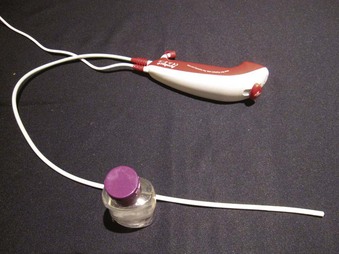
The Ambu aScope (Ambu A/S, Ballerup, Denmark) is a fully disposable, sterile, battery-operated non-FOB with a small (0.8-mm) working channel capable of drug instillation but not suctioning. It has a CMOS chip, a steering button to flex the tip, and a distal LED (Fig. 19-5). Images are transmitted along a cable within the insertion tube and transferred to a small portable monitor by way of a video connection cable at the handle. In its early version, this non-FOB had a timing mechanism allowing it to function for a maximum of 30 minutes during an 8-hour period starting from the initial power startup. A more recent version of this device comes without these time limitations. The monitor can be used for 150 intubations and is connected to an external monitoring system. Apart from routine use of this instrument, a particular advantage may occur in patients with highly contagious disease (e.g., Creutzfeldt-Jakob), in immunosuppressed patients, or in situations in which cheaper devices may be beneficial (e.g., where economic factors limit purchases or where FOB is seldom used).37,38
III Rationale for Fiberoptic Intubation
Airway management failure is a leading cause of patient morbidity and mortality in closed claims analyses.39 During the period 1999 to 2005, failed intubation or DI was the cause of 2.3% of the 2211 anesthesia-related deaths in the United States.40 With rigid laryngoscopes (RLs), DI was reported to have a frequency ranging from 1% to 13%.41–45 Shiga and colleagues performed a meta-analysis of studies on 50,760 patients and estimated the average occurrence of difficult intubation to be 5.8%,46 with a range of 4.5% to 7.5% overall47,48 and an even greater incidence in obstetric or obese patients.49–51 Anesthesia Associates, Inc. (AincA), San Marcos, CA Benumof’s estimate of “cannot intubate, cannot mask-ventilate” scenario was verified by Heidegger and associates at 0.007%,52,53 whereas Kheterpal and coworkers showed the incidence of impossible mask ventilation to be 0.15% in a prospective series of 53,041 patients.54
FOB intubation has always had a place in these scenarios, but this simple technique seems formidable to many. However, despite the increasing availability of less expensive intubating supraglottic airways (SGAs), lighted stylets, video laryngoscopes (VLs), and optical laryngoscopes (OLs), an explosion of improved laryngoscopic views, and greater intubating success rates, there is still a definite need for FOB intubation. A survey of American anesthesiologists by Rosenblatt and colleagues revealed a strong preference for FOB intubation for DA patients.55 Avarguès and associates’ survey of French anesthetists in 1999 revealed that 64% of responders expressed the need for more training in FOB intubation.56 Furthermore, FOB use was mentioned twice in the revised 2003 DA algorithm by a panel of experts who formulated the American Society of Anesthesiologists (ASA) Practice Guidelines for Difficult Airway Management.57 In a 2009 review of the existing algorithms for DA management, Frova and Sorbello affirmed that FOB is universally recognized as the gold standard in the awake, sedated, or anesthetized “difficult to intubate” patient.58
Finally and more practically, there have been numerous cases in which older devices and even the most recently developed airway management devices have proved to be inadequate. FOB employment and assistance steadfastly facilitates the execution of successful intubations, often rescuing these failures, either alone or as part of a multimodal approach to the DA.59,60
The FOB has a number of unique, wide-ranging characteristics:
• Its flexible, gliding attributes allow FOB skimming along the most twisted of DA obstacle courses while still posting the highest success rates for intubation.
• During intubation, the FOB is the device least likely to result in any cervical spine motion.61
• The FOB can be used for intubation in any body position, via oral or nasal routes, in all age groups.
• Under excellent local airway anesthesia, FOB intubation is associated with negligible hemodynamic changes.
• The FOB has multiple diagnostic capabilities for determining abnormal anatomy, assisting with implementation of other airway devices, and trouble-shooting ventilatory problems.
A Indications for Flexible Fiberoptic or Endoscopic Intubation
Although many anesthesiologists routinely carry out FOB intubation on all of their patients, most tend to adhere to specific indications. There is no hard or fast rule for “awake” versus “asleep” fiberoptic intubation. An awake approach is usually chosen to lessen significant patient risk in comparison to other methods, especially rigid laryngoscopy. The awake approach may be more advisable in cases of a very difficult airway or with a somewhat difficult airway in a patient with intolerant comorbidities endangered by the trauma or hypoxemia of non-FOB techniques (e.g., critical coronary artery disease). A list of indications for flexible airway endoscopy is presented in Box 19-1.
Box 19-1 Indications for Use of Flexible Fiberoptic or Endoscopic Intubation
3. Prevention of cervical spine motion in at-risk patients
4. Avoidance of traumatic oral or nasal effects of intubation (e.g., loose teeth, nasal polyps)
5. Avoidance of aspiration in high-risk patients
7. Therapeutic uses beyond planned FOB intubation
FOB, Fiberoptic bronchoscope; SGA, supraglottic airway device.
B Contraindications: Absolute, Moderate, and Relative
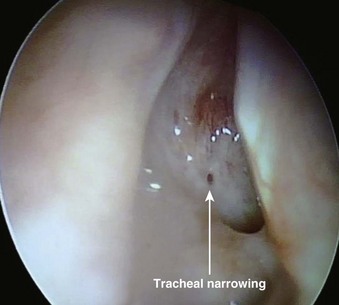
The most important contraindication to FOB use may well be lack of skill of the endoscopist (i.e., FOB operator) due to inadequate training or inadequate maintenance of formerly acquired skills. Any lack of a trained assistant or of available, ready to use, proper equipment may also negate FOB plans. There are few other contraindications to FOB use. These may involve situations in which harm to the instrument may occur (e.g., uncooperative patients), cases in which FOB use is extremely likely to be unsuccessful (e.g., patients with known near-total upper airway obstruction [Fig. 19-6]), or rare instances in which another technique may pose less risk for airway management (e.g., patients who have massive facial trauma with excessive bleeding might do better with a tracheostomy). Contraindications for awake and asleep FOB intubation are listed in Boxes 19-2 and 19-3, respectively.
Box 19-2 Contraindications for Awake FOB Techniques
ENT, Ear, nose, and throat; ETT, endotracheal tube; FOB, fiberoptic bronchoscope.
Box 19-3 Contraindications for Asleep FOB Techniques
ENT, Ear, nose, and throat; ETT, endotracheal tube; FOB, fiberoptic bronchoscope.
IV Equipment
A Fiberoptic and Non-fiberoptic Bronchoscope Model Details
FOB specifications and characteristics have been presented in Table 19-1. Some models have eyepieces or video attachments. Others have varying degrees of portability. Sizes and ranges of view are also variable, from the smallest infant to the largest adult size. In 2010, Olympus developed a unique MAF-GM system; it features a lithium rechargeable battery-operated FOB with a fixed, rotatable video screen by the handle––all totally immersible for sterilization. Its CCD camera has a memory card and an xD chip for still photography and video recording.
B Fiberoptic Bronchoscope Cart
Preparation for highly useful FOB techniques is important to increase the likelihood of success. The incorporation of an FOB cart as a movable asset capable of rapid transport to operating rooms, specialty units, or floors is ideal for routine or emergency use. Contents need to be arranged logically in a readily available design so that the composition and organization will be uniform across the facility in the event that more than one cart is needed for an institution. All airway specialists must be familiar with the contents of the FOB cart and know how to have it transported to required patient sites without delay. Preferably, the cart should have two widely separated tubular structures for hanging a clean FOB and, later, a used one (Fig. 19-7). Typically, the FOB cart will also have a light source, a video monitor (ideally), endoscopy masks, bronchoscopy swivel adapters, oral intubating airways, bite blocks, atomizers, tongue blades, cotton-tipped swabs, gauze, soft nasal airways, and local anesthetics (e.g., 2% and 4% lidocaine, 2% lidocaine gel or paste).
Optionally, an FOB cart setup could also be incorporated as part of a more complete DA cart that includes items needed to follow the DA algorithm. Among other items, a DA cart should contain a VL and screen, SGAs, intubating SGAs, ETT introducer/exchangers,62,63 and percutaneous airway rescue sets.
C Ancillary Equipment
1 Bronchoscopy Swivel Adapters and Endoscopy Masks
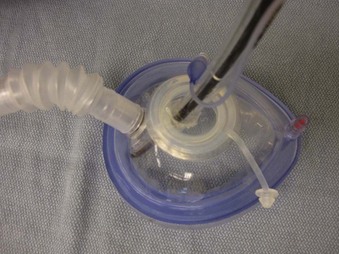
Bronchoscopy swivel adapters have the capacity to rotate when placed between the ventilating system and a mask, SGA, or ETT. They look like elbow adapters but are equipped with a “flip-cap port” covering a diaphragm with a central opening (Fig. 19-8). This permits passage of an FOB with virtually no leak during use of the continuous ventilation system on a patient.
Endoscopy masks differ from regular masks by having at least one larger additional opening whose function is similar to that of a bronchoscopy swivel adapter port (Fig. 19-9). The opening permits both FOB and ETT passage for intubation during continuous mask ventilation. Some masks have recessed one-way, valve-like openings for FOB and ETT entry.
2 Intubating Oral Airways
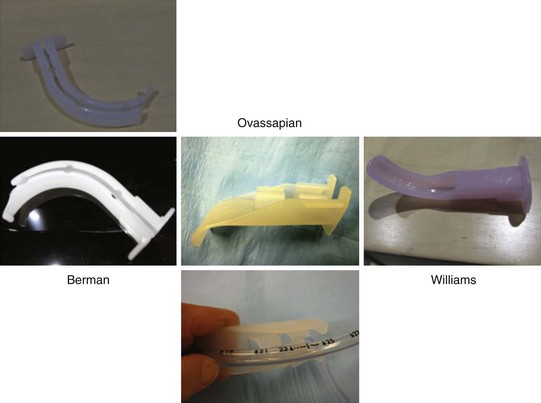
Intubating oral airways (IOAs) are often used in unconscious patients or in the topically anesthetized oropharynx of awake patients to act as a conduit through which an FOB is inserted for oral intubation (Fig. 19-10). It is extremely useful when an assistant holds the IOA midline throughout the intubation procedure. These airways should not be jammed fully into the patient if they seem too large for smaller mouth openings. If no IOA is used secondary to inadequate mouth opening or endoscopist preference, a bite block should be placed between the molars or incisors to prevent FOB injury.

Full access? Get Clinical Tree