Extraglottic Devices
Erik G. Laurin
Michael F. Murphy
INTRODUCTION
The term extraglottic device (EGD), and its subclasses, supraglottic device (SGD), infraglottic device (IGD), and retroglottic device (RGD) are defined and discussed in Chapter 10. This chapter reviews the non-laryngeal mask airway (LMA) EGDs, all of which can be classified as RGDs, our preferred term, or, less precisely, as IGDs. For the balance of this chapter, we refer to these devices collectively as EGDs, recognizing that the LMAs, a distinct class of EGDs, have been discussed in Chapter 10. The uses and contraindications of these retroglottic EGDs, however, are comparable to those for the supraglottic (LMA-type) EGDs, and are discussed in Chapter 10. These devices share the following contraindications with the SGDs:
Responsive patients with intact airway-protective reflexes
Patients with known esophageal disease
Caustic ingestions
Upper airway obstruction because of laryngeal foreign bodies or pathology
Modern EGDs represent a dramatic improvement over the esophageal obturator airway and the esophageal gastric tube airway of the 1970s and 1980s, which have no place in modern emergency airway management. Instead, several of these devices have demonstrated their effectiveness and safety in rapidly establishing oxygenation and ventilation in a variety of emergency situations. This chapter will focus on the devices that have sufficient data to support their use in emergency airway management, as opposed to nonemergency operating room cases. Some of the currently available EGDs that lack a track record in emergency airway practice will require more evaluation before their potential role can be understood.
RETROGLOTTIC DEVICES
To many practitioners, the most familiar EGD is the esophageal tracheal Combitube (Esophageal-Tracheal Combitube (ETC) or Combitube [Tyco-Healthcare-Kendall-Sheridan, Mansfield, MA]). It has been in use since 1987, and has substantial evidence supporting its use. It creates a seal above and below the laryngeal inlet, and provides a direct conduit for gas exchange that is easier to use and more effective than a bag and a mask, and less invasive than an endotracheal tube (ETT) in that it is not intended to enter the trachea. The success of the Combitube has spawned the development of devices based on the same principles, attempting to replicate or improve on its safety, ease of use, and ability to facilitate oxygenation and ventilation.
The devices in this class commonly are collectively called EGDs, or, more precisely, RGDs, and include the following:
ETC or Combitube (Fig. 11-1)
King LT airway (King Systems Corp, Noblesville, IN; also known as laryngeal tube airway) (Fig. 11-2)
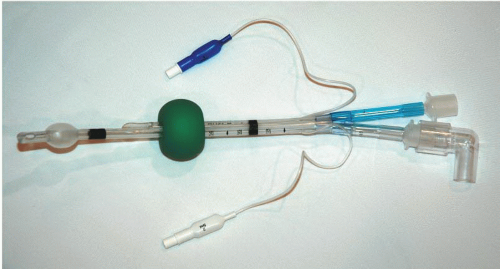
Rusch EasyTube (Fig. 11-3)
LaryVent (LV Teleflex Medical Rusch, Research Triangle Park, Raleigh, NC, USA)
Airway management device (AMD)
These devices are intended to be passed behind the larynx and glottis (hence, retroglottic) and inserted blindly into the esophagus. Each is designed with two high-volume, low-pressure cuff balloons. One balloon seals the oropharynx above and the other the esophagus below, trapping the laryngeal inlet between the two. The Combitube and the EasyTube use two separate inflation ports to enable independent balloon inflation; the King LT has a single inflation port that inflates both the upper and lower portions of the balloon. Two somewhat similar devices, the LV and the AMD, have not achieved a body of evidence at the time of writing to recommend their use in emergency airway management and are therefore not further discussed.
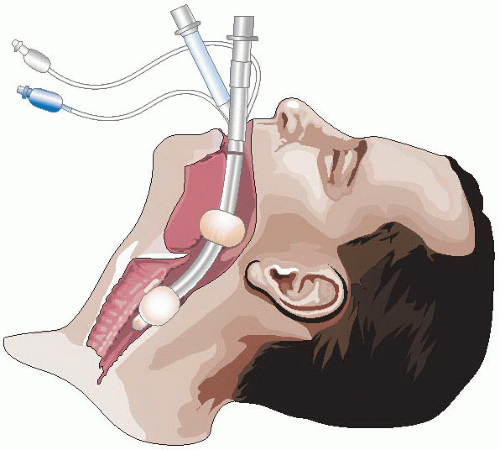 Figure 11-1 • The Combitube Inserted and Seated. Note how the laryngeal aperture is trapped between the two balloons. |
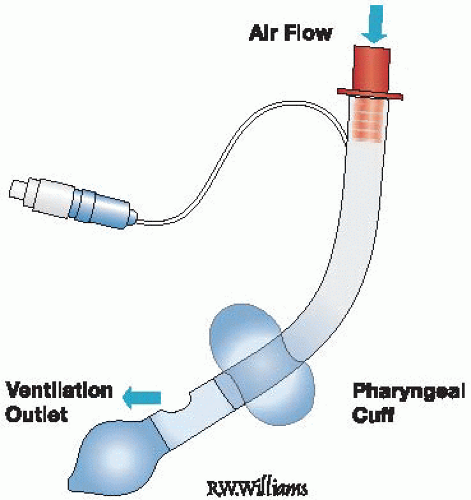 Figure 11-2 • The King LT Airway. Note that there is only one inflation port to inflate both balloons. |
When compared with LMAs, some of these EGDs may offer advantages. For instance, these balloon-type devices typically have higher cuff leak pressures (up to 40 cm H2O) than LMA-type devices (up to 25 cm H2O), which may be advantageous in patients requiring high peak airway pressures (asthma and obesity) or if glottic anatomy is distorted from hematoma, infection, or mass,
requiring increased inflation pressure. None of these EGDs, however, is specifically designed to be a conduit through which a definitive airway can be established, like the intubating LMA.
requiring increased inflation pressure. None of these EGDs, however, is specifically designed to be a conduit through which a definitive airway can be established, like the intubating LMA.
Combitube
The Combitube (Fig. 11-1) has been in clinical use for a much longer period of time than any of the other EGDs has, and therefore it has accumulated the largest body of evidence describing its indications, contraindications, benefits, and risks.
The Combitube is a dual-lumen, dual-cuff, disposable airway intended to be inserted into the esophagus, although it can rarely enter the trachea on insertion (<5% of insertions), and is designed to function adequately in either position. The Combitube is supplied in two sizes: 37F SA (small adult) to be used in patients 4 ft (1.22 m) to 5 ft 6 in (1.67 m) tall, and 41F, which is for use in patients more than 5 ft 6 in tall. There is no Combitube suitable for use in children or patients <4 ft tall. In addition to the indications listed earlier, use of the Combitube has been described in upper gastrointestinal or upper airway hemorrhage that threatens airway and tracheal patency and in a case of severe facial burns.
Insertion Technique
Insertion of the Combitube is a blind technique, although a laryngoscope may be used, permitting insertion under direct vision. Although the Combitube can be inserted in almost any conceivable patient position, including sitting, semi-prone, etc., the technique described here assumes the patient is in the supine position.
1. With the patient supine and the head and neck in a neutral position, lift the tongue and jaw upward (jaw lift) with the nondominant hand.
2. Insert the device in the midline, allowing the curve of the device to follow the natural curve of the airway, and advance the device until the upper incisors (or alveolar ridge if the patient is edentulous) lie between the imprinted black circular bands on the device. Moderate force is required to enable the device to pass through the pharyngeal constrictor muscles into the esophagus. Substantial resistance should prompt the operator to withdraw and readvance.
3. Inflate the proximal large oropharyngeal balloon with approximately 100 ml of air (Combitube SA: 85 ml) through the blue pilot balloon port labeled no. 1.
4. Inflate the white distal balloon with 5 to 15 ml of air (Combitube SA: 5 to 12 ml) through the white pilot balloon port labeled no. 2.
5. Begin ventilation using the longer blue connecting tube (labeled no. 1). The presence of air entry into the lung, the detection of end-tidal carbon dioxide, and the absence of gastric insufflation by auscultation indicate that the Combitube is in the esophagus, which occurs with virtually every insertion. With the Combitube in the esophageal position, aspiration of gastric contents and gastric decompression is possible by passing the provided suction catheter through the clear connecting tube (labeled no. 2) into the stomach.
6. If bag ventilation using the longer blue tube no. 1 results in no breath sounds in the chest, absence of end-tidal carbon dioxide detection, and the presence of gastric insufflation sounds, then the Combitube is in the trachea (a distinctly rare event), and ventilation should be performed through the shorter clear connection tube no. 2.
7. The absence of any sounds on auscultation may indicate that the device has been inserted too far and should be repositioned after the proximal balloon is deflated.
After the Combitube is placed in the esophagus, oxygenation and ventilation can occur, but there is no way to establish a definitive airway when the device is in use. To intubate, the operator has to deflate and remove the Combitube entirely, or deflate just the proximal balloon and intubate around the Combitube, which remains in place in the esophagus. If the Combitube is placed in the trachea, an ETT changer or gum-elastic bougie (i.e., intubating introducer) can be placed through the ventilating tube and down the trachea, the Combitube balloons deflated and device removed, and a standard ETT advanced over the tube changer into the trachea. Even with tracheal placement of the Combitube, it is advisable to exchange it with a standard ETT within hours because of concerns of mucosal ischemia from the firm walls of the Combitube against the pharynx, even with the pharyngeal cuff deflated.
Complications
The Combitube has been shown to be an effective device that is easy to position properly. The Combitube appears in one study to be superior to the LMA Classic in the prehospital setting, and it has been shown to be a useful airway rescue device in the event of a failed intubation. However, like the LMA, it does not provide optimum protection against aspiration (although aspiration is uncommon), and its merit relative to the LMA Fastrach is unknown. Complications are rare and mostly related to upper airway and esophageal hematomas, mucosal lacerations on insertion, pyriform sinus perforation, and perforation of the esophagus.
Increased cuff volumes are required at times to achieve a seal sufficient to permit adequate ventilation. As cuff volume is increased, the pressure transmitted to the mucosa is increased to the point, where mucosal perfusion may be compromised, particularly where the cuff is adjacent to rigid anatomical structures such as the cervical spine (pharyngeal balloon) and the larynx (esophageal balloon). Over time, this may lead to ischemic mucosal injury. A high rate of success with few complications has been reported in prehospital use for cardiac arrest victims.
Finally, it should be noted that the pharyngeal balloon on the Combitube (as opposed to the Rusch EasyTube or the King LT airway) is made of latex.
The King LT Airway
The King LT airway (also known as the laryngeal tube airway, predominantly in Europe) (Fig. 11-2) is a more recently developed multiuse (King LT) and disposable (King LT-D), latexfree, single-lumen silicon laryngeal tube with oropharyngeal and esophageal low-pressure cuffs, with a ventilation port located between the two cuffs. It is supplied in blind distal tip (King LT, LT-D) and open distal tip (King LTS, LTS-D) variants to permit gastric decompression. A single pilot balloon port is used to inflate both balloons simultaneously, which is technically easier to use
than the two-step Combitube inflation system. However, this single inflation technique also allows the King LT’s airway seal to sometimes be lost following insertion, requiring deflation of the balloons and repositioning, an occurrence that is not common with the Combitube.
than the two-step Combitube inflation system. However, this single inflation technique also allows the King LT’s airway seal to sometimes be lost following insertion, requiring deflation of the balloons and repositioning, an occurrence that is not common with the Combitube.
The King LT is inserted similarly to the Combitube, although there is usually less resistance on insertion. The distal segment of the tube is straight instead of slightly curved like the Combitube, so the King LT is designed to go only into the esophagus, with no reported tracheal placements. It is advanced until definitive resistance is felt, or the colored, 15/22-mm bag connector flange touches the incisors. When seated, it works in a fashion that is very similar to that of the Combitube, with ventilation through the ports between the two cuff balloons. Because there is only one ventilation bag adaptor and one pilot balloon inflation port, and the device never enters the glottis or trachea, troubleshooting is rarely needed. If the first attempt at ventilation after insertion results in no air movement and high resistance to ventilation, the tube is too deep and can be slowly withdrawn with continued pressure on the ventilation bag, until ventilations are free flowing. Ventilation and oxygenation capabilities seem to be similar to the LMA and Combitube.
As is the case with the Combitube, sizing of the King LT airways is height based. The King LT is available for pediatric and adult patients from 35 in (90 cm) to more than 6 ft (>180 cm) tall. Approximate interdental distance of 20 mm is required for insertion, comparable to the Combitube.
Although it is possible to intubate using the King LT, caution is required as complications have been reported in a cadaver model. The 10 mm inner diameter of the ventilation lumen can accommodate a gum-elastic bougie, which can then exit the ventilation port at the distal end of the lumen. Ideally, this ventilation port lines up with the glottic opening, allowing the bougie to enter the trachea with the typical confirmatory “clicks” on the tracheal rings and “hard stop” with advancement into a small bronchus. The King LT can then be removed and a standard ETT advanced over the bougie into the trachea. Although this procedure can sometimes be accomplished in manikins, there is potential for bougie perforation of glottic structures during advancement through the King LT in human tissue. Therefore, if this procedure is attempted, extreme care must be exercised.
Rusch EasyTube
The Rusch EasyTube (Fig. 11-3) is a dual-lumen tube designed for difficult or emergency airway intubation and ventilation. Like the Combitube, the EasyTube can be placed either in the trachea or in the esophagus, and creates a viable airway in either position. When placed in the esophagus, the EasyTube allows the passage of a flexible endoscope, a suction catheter, or a tracheal tube introducer through the proximally terminating ventilation lumen. This distinguishes it from the Combitube, which does not permit passage of a tube exchanger to potentially establish a definitive airway through the device. If placed in the trachea, the size and shape of the distal tip are similar to a standard ETT. It is suggested by the manufacturer that the risk of tracheal trauma relative to the Combitube is reduced because of the smaller diameter of EasyTube device at the distal tip.
The EasyTube is supplied in two sizes, 28F and 41F. As for the Combitube, the manufacturer claims that the smaller size can be used in older children. The EasyTube is latex free.
There is minimal evidence from human studies to demonstrate the relative success rate of the EasyTube versus the LMA, Combitube, or King LT, although initial data appear promising. Multiple manikin studies show that it is similar to a Combitube in speed of insertion, successful ventilations, and skill retention. More data are needed to determine its role in emergency airway management.
EVIDENCE
What is the role of the Combitube as a rescue airway device? The Combitube has been identified as a rescue airway device for the failed airway by authoritative bodies in the United States and Canada.1,2 Its use is well described in the anesthesia, resuscitation, emergency
medicine, and emergency medical services (EMS) literature both as a first-line device and as a device to be used in the face of a difficult or failed airway.3,4,5,6,7,
Full access? Get Clinical Tree