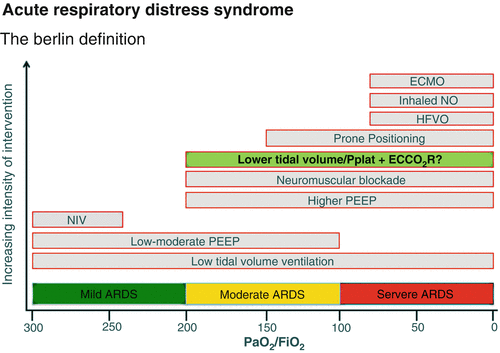
Fig. 82.1
Admission Chest Radiograph
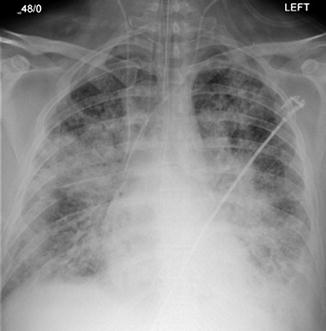
Fig. 82.2
Hospital day 2 Chest Radiograph
Question
What intervention would you consider next?
Answer
Extracorporeal membrane oxygenation (ECMO) evaluation
This patient has severe ARDS (paO2/FiO2 ratio < 100) [1] and was initiated on lung protective ventilation measures per the ARDSnet strategy [2]. With the development of severe hypoxemia, appropriate rescue strategies were implemented (proning, neuromuscular blockade, recruitment maneuvers, and inhaled nitric oxide). When severe refractory hypoxemia developed, he was evaluated for ECMO. A bicaval dual lumen ECMO cannula was placed via the right internal jugular vein under serial X-ray guidance [3] (Figs. 82.3 and 82.4).
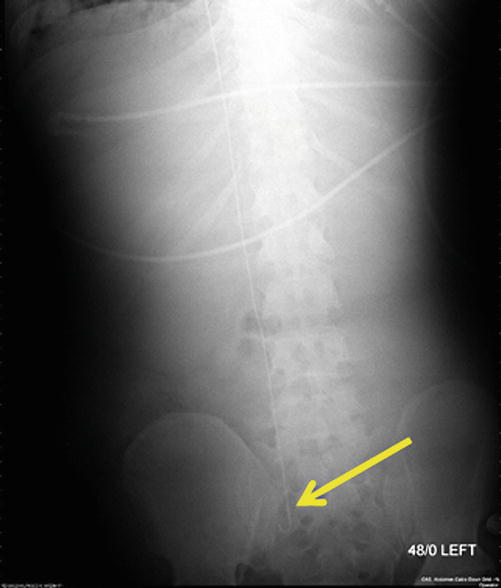
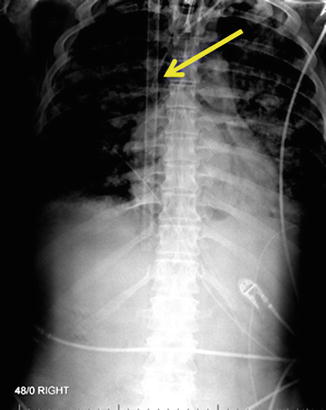

Fig. 82.3
Guidewire from right IJ to right iliac vein

Fig. 82.4
31-French Bicaval Dual-lumen Cannula for VV-ECMO via right IJ vein
A heparin bolus (100 u/kg IV) was administered and a 31 French bicaval dual-lumen cannula was advanced into position with the tip in the subdiaphragmatic, perihepatic inferior vena cava (Figs. 82.3, 82.4, and 82.5). Venovenous ECMO was initiated with a sweep of 2 L/min on a single oxygenator and a blood flow rate of 4 L/min on the centrifugal pump. His ABG on these settings were 7.38/42/75. Continuous heparin infusion was initiated for systemic anticoagulation. FiO2 was weaned to 50 %, inhaled nitric oxide weaned off, neuromuscular blockade was discontinued, and spontaneous ventilation was initiated. VV-ECMO was weaned when native lung oxygenation improved.
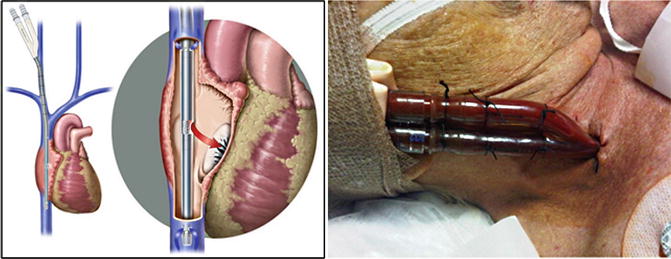

Fig. 82.5
Optimal position of the bicaval dual-lumen cannula with de-oxygenated blood outflow to ECMO circuit and oxygenated blood inflow to right atrium via medial infusion port
Principles of Management
ECMO Indications
ECMO should be considered for the most severe forms of acute respiratory failure, including severe hypoxemia and severe ARDS when other less costly strategies fail (Fig. 82.6). ECMO replaces pulmonary function, allows ultra-protective mechanical ventilation settings, and may facilitate lung healing.
ECMO should be considered in hypoxic respiratory failure from any cause when the risk of mortality is 50 % or greater, and is indicated when the risk of mortality is 80 % or greater. A 50 % mortality risk is associated with a PaO2/FiO2 < 150 on FiO2 > 90 % and/or Murray score 2–3 (Table 82.1). An 80 % mortality is associated with a PaO2/FiO2 < 100 on FiO2 > 90 % and/or Murray score 3–4 despite optimal care for 6 h or more. Other indications include CO2 retention on mechanical ventilation despite high Pplateau (>30 cm H2O), severe air leak syndromes, a need for intubation in a patient on the lung transplant list, and immediate cardiac or respiratory collapse (PE, blocked airway, unresponsive to optimal care).
Table 82.1
Murray Score for consideration of ECMO
Parameter/score | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
PaO2/FiO2 (On 100 % Oxygen) | ≥300 mmHg ≥40 kPa | 225–299 30–40 | 175–224 23–30 | 100–174 13–23 | <100 <13 |
CXR | Normal | 1 point per quadrant infiltrated | |||
PEEP (cmH2O) | ≤5 | 6–8 | 9–11 | 12–14 | ≥15 |
Compliance (ml/cmH2O) | ≥80 | 60–79 | 40–59 | 20–39 | ≤19 |
ECMO survival in adults with severe respiratory failure has significantly improved. The ELSO registry report from 1986 to 2006 included 1473 patients with severe respiratory failure treated with ECMO and 50 % survived to hospital discharge [4]. In the most recent study of adult patients with severe acute respiratory failure treated with ECMO from 2000 to 2012 from the Extracorporeal Life Support Organization (ELSO) international registry, 1338 (57 %) of 2355 patients were discharged alive from hospital [5]. Hospital survival was 71 % in 2009 Influenza A (H1N1) ARDS patients treated with ECMO [6]. Referral to an ECMO Center was associated with significantly decreased mortality among patients with severe ARDS due to 2009 Influenza A (H1N1) viral pneumonia [7]. But it is recognized that data from retrospective series will never eliminate the bias of patient selection to receive ECMO.
Positive reports from the prospective randomized CESAR trial in 2009 (Conventional Ventilation or ECMO for Severe Adult Respiratory Failure trial) and from the H1N1 pandemic, in addition to reduced complexity and increased safety of ECMO have led to an increased use of ECMO for severe ARDS. The CESAR trial documented no death or severe disability in 63 % of patients randomized to the Leicester protocol, which included ECMO, compared to 47 % on conventional treatment [8]. The CESAR trial confirmed significant benefit for a strategy of referral to a single ECMO-capable hospital for ECMO assessment and management if criteria were met. Overall survival for adult respiratory ECMO is 57 % and since the H1N1 pandemic of 2009, and ECMO usage for adult respiratory failure remains above 400 cases per year [6, 9].
ECMO Contraindications
Although there are no absolute contraindications to ECMO, the following factors should be considered relative contraindications:
- 1.
Mechanical ventilation at high settings (FiO2 > 0.9, P-plat >30 cm H2O) for 7 days or more (Lower likelihood of recovery if >5 days on the ventilator at these settings)
- 2.
Major pharmacologic immunosuppression (absolute neutrophil count <400/mm3)
- 3.
Contraindications to systemic anticoagulation (includes recent or expanding CNS hemorrhage)
Full access? Get Clinical Tree