Epidural and Spinal Anesthesia
There are no absolute indications for spinal or epidural anesthesia (Bernards CM, Hostetter LS. Epidural and spinal anesthesia. In: Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Ortega R, Stock MC, eds. Clinical Anesthesia. Philadelphia: Lippincott Williams & Wilkins; 2013:905–934). Spinal anesthesia and epidural anesthesia have been shown to blunt the “stress response” to surgery, decrease intraoperative blood loss, lower the incidence of postoperative thromboembolic events, possibly decrease morbidity in high-risk surgical patients, and serve as a useful method to extend analgesia into the postoperative period (better analgesia than can be achieved with parenteral opioids).
I. Anatomy
Proficiency in spinal and epidural anesthesia requires a thorough understanding of the anatomy of the spine and spinal cord.
Vertebrae
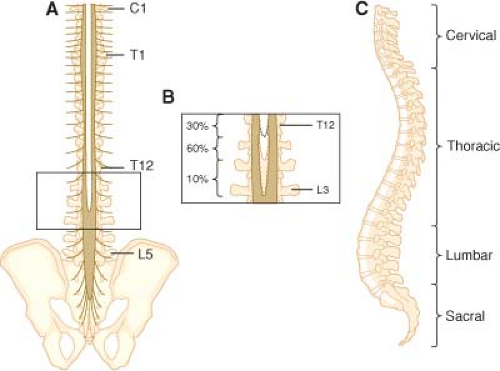
The spine consists of 33 vertebrae (7 cervical, 12 thoracic, 5 lumbar, 5 fused sacral, and 5 fused coccygeal).
With the exception of C1 (which lacks a body or spinous process), the vertebrae consist of a body anteriorly; two pedicles that project posteriorly from the body; and two lamina that connect the pedicles to form the vertebral canal, which contains the spinal cord, spinal nerves, and epidural space (Fig. 34-1).
The laminae give rise to the transverse processes, which project laterally, and the spinous process, which projects posteriorly (see Fig. 34-1).
The fifth sacral vertebra is not fused posteriorly, giving rise to a variably shaped opening known as the sacral hiatus (opening into the sacral canal, which is the caudal termination of the epidural space). The sacral cornu are bony prominences on either side to the hiatus and aid in identifying it.
Identifying individual vertebrae is important for correctly locating the desired interspace for performance of epidural and spinal anesthesia (Table 34-1).
Ligaments
The vertebral bodies are stabilized by five ligaments that increase in size between the cervical and lumbar vertebrae (see Fig. 34-1).
The ligamentum flavum is thickest in the midline (3–5 mm at L2–L3) and also farthest from the spinal meninges in the midline (4–6 mm at L2–L3). As a result, midline insertion of an epidural needle is least likely to result in accidental meningeal puncture.
Epidural Space
The epidural space lies between the spinal meninges and the sides of the vertebral canal. It is bounded cranially by the foramen magnum, caudally by the sacrococcygeal ligament (sacral hiatus), and posteriorly by the ligamentum flavum and vertebral pedicles.
The epidural space is not a closed space but communicates with the paravertebral space via the intervertebral foramina.
The epidural space is composed of a series of discontinuous compartments, which become continuous when the potential space separating the compartments is opened up by injection of air or liquid.
The most ubiquitous material in the epidural space is fat.
Veins are present principally in the anterior and lateral portions of the epidural space with few, if any, veins present in the posterior epidural space (see Fig. 34-1). These veins anastomose freely with extradural veins (pelvic veins, azygous system, intracranial veins).
Epidural fat has important effects on the pharmacology of epidurally and intrathecally administered opioids and local anesthetics.
Lipid solubility results in opioid sequestration in epidural fat with associated decreases in bioavailability.
Transfer of opioids from the epidural space to the intrathecal space is greatest with poorly lipid-soluble morphine and least for the highly lipid-soluble opioids fentanyl and sufentanil.
Meninges
Dura mater is the outermost and thickest meningeal tissue that begins at the foramen magnum (fuses with the periosteum of the skull, forming the cephalad border of the epidural space) and ends at approximately S2, where it fuses with the filum terminale. The dura mater extends laterally along the spinal nerve roots and becomes continuous with the connective tissue of the epineurium at approximately the level of the intervertebral foramina.
The inner edge of the dura mater is highly vascular, which likely results in the dura mater being an important route of drug clearance from both the epidural and subarachnoid space.
The presence of a midline connective tissue band (plica mediana dorsalis) running from the dura mater to the ligamentum flavum is controversial but may be invoked as an explanation for unilateral epidural block.
The subdural space is a potential space between the dura mater and arachnoid mater. Drug intended for either the epidural space or the subarachnoid space may be accidentally injected into this space.
Arachnoid Mater
The arachnoid mater is an avascular membrane that serves as the principal physiologic barrier for drugs moving between the epidural space and the subarachnoid space.
The subarachnoid space lies between the arachnoid mater and pia mater and contains cerebrospinal fluid (CSF). The spinal CSF is in continuity with the cranial CSF and provides an avenue for drugs in the spinal CSF to reach the brain. Spinal nerve roots and rootlets run in the subarachnoid space.
Pia mater is adherent to the spinal cord.
CSF (100–160 mL in adults, produced at a rate of 20–25 mL/hr) is replaced roughly every 6 hours (removed by arachnoid villi).
Contrary to a widely held view, CSF does not circulate through the subarachnoid space but rather oscillates in parallel with cerebral expansion and contraction during the cardiac cycle. (Net CSF movement is estimated to be 0.04% per oscillation.)
CSF cannot be relied on to distribute drugs in the subarachnoid space.
The kinetic energy of the injection and baricity of the solution serve to distribute drug during a single-shot spinal.
The lack of significant net CSF motion explains why drug distribution during slow infusions used for chronic intrathecal analgesia results in limited drug distribution.
Spinal Cord
In adults, the caudad tip of the spinal cord typically ends at the level of L1 (extends to L3 in 10% of adults).
The spinal cord gives rise to 31 pairs of spinal nerves, each composed of an anterior motor root and a posterior sensory root.
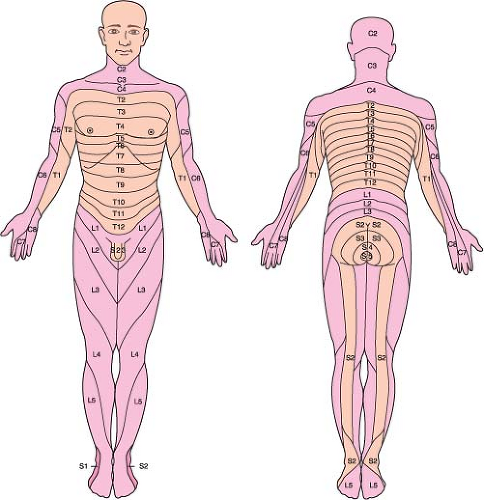
Dermatome is the skin area innervated by a given spinal nerve (Fig. 34-2).
The intermediolateral gray matter of T1 to L12 contains the cell bodies of the preganglionic sympathetic neurons. These sympathetic neurons travel with the corresponding spinal nerve to a point just beyond the intervertebral foramen, where they exit to join the sympathetic chain ganglia.
Because the spinal cord ends between L1 and L2, the thoracic, lumbar, and sacral nerve roots travel increasingly longer distances in the subarachnoid space (cauda equina) to reach the intervertebral foramen through which they exit.
Table 34-1 Landmarks for Vertebral Interspaces | ||||||||
|---|---|---|---|---|---|---|---|---|
|
II. Technique
Spinal and epidural anesthesia should be performed only after appropriate monitors are applied in a setting in which equipment for airway management and resuscitation is immediately available.
Needles
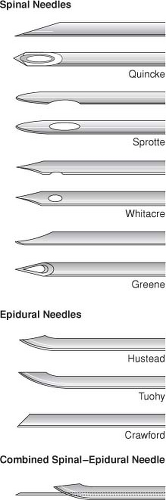
Spinal and epidural needles are named for the design of their tips (“pencil point,” beveled tip with cutting edge) (Fig. 34-3).
Epidural needles have a larger diameter than spinal needles, facilitating injection of air or fluid for the “loss of resistance” technique and passage of catheters.
The outside diameters of spinal and epidural needles are used to determine their gauges. Large-gauge spinal needles (22–29 gauge) are often easier to insert if an introducer (inserted into the interspinous ligament) is used. Postdural puncture headache (PDPH) is less likely when small-gauge spinal needles are used.
All spinal and epidural needles come with a tight-fitting stylet to prevent the needle from becoming plugged with skin or fat.
Sedation before placement of the block is limited because patient cooperation (positioning, determination of level of sensory anesthesia, occurrence of paresthesias) is important. After the anesthesia is established, the patient may be sedated as deemed appropriate.
III. Spinal Anesthesia
Position (Table 34-2)
In the lateral decubitus position, the patient lies with the operative side down when hyperbaric solutions are being used. The patient’s shoulders and hips are positioned perpendicular to the bed (preventing rotation of the spine), the knees are drawn up to the chest, the neck is flexed, and the patient is asked to actively curve the back outward (which spreads apart the spinous processes).
Using the iliac crests as landmarks, the L2 to L3, L3 to L4, and L4 to L5 interspaces are identified and the desired interspace chosen.
All antiseptic solutions are neurotoxic, and care must be taken not to contaminate spinal needles and local anesthetics. Chlorhexidine–alcohol antiseptic prevents colonization of percutaneous catheters better than 10% povidone–iodine and is the recommended prep for skin asepsis before regional anesthesia procedures.
Midline Approach
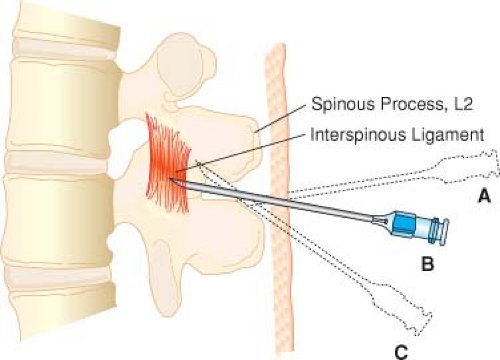
After infiltration of the selected needle insertion site with local anesthetic solution, the needle is advanced (subcutaneous tissue to supraspinous ligament to interspinous ligament to ligamentum flavum to epidural space to dura mater [“pop”] to arachnoid mater) until CSF is obtained (gentle aspiration may be helpful). The spinal meninges are typically at a depth of 4 to 6 cm.
If bone is encountered, the depth should be noted and the needle withdrawn to subcutaneous tissue and redirected more cephalad (Fig. 34-4).
If the patient experiences paresthesia (which should be differentiated from discomfort caused by contacting
bone), it is important to immediately stop advancing the needle and determine whether the needle tip has encountered a nerve root in the epidural space or in the subarachnoid space. (The presence of CSF confirms that the needle has encountered a cauda equina nerve root.)
After completing the injection of local anesthetic solution, a small volume of CSF is again aspirated to confirm that the needle tip has remained in the subarachnoid space.
After the block has been placed, strict attention must be directed to the patient’s hemodynamic status with blood pressure and heart rate supported as necessary.
The level of anesthesia should be assessed by pinprick or temperature sensation. If the anesthesia is not rising high enough, the table may be tilted to influence spread of a hyperbaric or hypobaric local anesthetic.
The paramedian approach is used when the patient cannot flex the spine or heavily calcified interspinous ligaments. The needle is inserted 1 cm lateral to the desired interspace with advancement toward the midline. (The first significant resistance is the ligamentum flavum as the interspinous ligament is bypassed.)
The lumbosacral approach is a paramedian approach directed at the L5 to S1 interspace.
Table 34-2 Patient Position for Performance of Spinal Anesthesia | |||
|---|---|---|---|
|
IV. Continuous Spinal Anesthesia
The technique is similar to that used for single-shot spinal anesthesia except that a needle large enough to accommodate the desired catheter must be inserted. (The catheter is inserted 2 to 3 cm into the subarachnoid space.)
Although smaller catheters decrease the risk of PDPH, they have been associated with reports of neurologic injury. (The recommendation is to avoid using a catheter smaller than 24 gauge.)
V. Epidural Anesthesia
Patient preparation and positioning, the use of monitors, and the needle approaches for epidural anesthesia are the same as for spinal anesthesia. However, unlike spinal anesthesia, epidural anesthesia may be performed at any intervertebral space.
Using the midline approach, the epidural needle is inserted into the interspinous ligament (“gritty” feel)
and then advanced slowly until the ligamentum flavum is contacted (increased resistance).
The epidural needle must traverse the ligamentum flavum and stop in the epidural space (“loss of resistance”) before encountering the spinal meninges.
A glass syringe containing 2 to 3 mL of saline and 0.1 to 0.3 mL of air is attached to the epidural needle, and the plunger is pressed. If the needle is properly placed in the ligamentum flavum, it will be possible to compress the air bubble without injecting the saline. If the air bubble cannot be compressed without injecting fluid, then the needle tip is most likely not in the ligamentum flavum but instead in the interspinous ligament or off midline in the paraspinous muscles.
After the ligamentum flavum is identified, the needle is slowly advanced with the nondominant hand while the dominant hand maintains constant pressure on the syringe plunger (Fig. 34-5).
As the needle enters the epidural space, there will be a sudden and dramatic loss of resistance as the saline is rapidly injected. (The patient should be warned of
Full access? Get Clinical Tree