Endoscopic Placement of Feeding Tubes
Lena M. Napolitano
Indications for Enteral Feeding
Nutritional support is an essential component of intensive care medicine. It has become increasingly evident that nutritional support administered via the enteral route is far superior to total parenteral nutrition [1,2,3,4,5,6,7,8,9 and 10]. The Canadian Clinical Practice Guidelines for Nutrition Support in Critically Ill Adults [1], the European Society for Clinical Nutrition and Metabolism (ESPEN) Guidelines on Enteral Nutrition for Intensive Care [2], and the Practice Management Guidelines for Nutritional Support of the Trauma Patient [3] all strongly recommend that enteral nutrition be used in preference to parenteral nutrition. An evidence-based consensus statement on the management of critically ill patients with severe acute pancreatitis also recommended that enteral nutrition be used in preference to parenteral nutrition [11]. A more recent systematic review also concluded that patients with severe acute pancreatitis should begin enteral nutrition early because such therapy modulates the stress response, promotes more rapid resolution of the disease process, and results in better outcome [12].
Although there are absolute or relative contraindications to enteral feeding in selected cases, most critically ill patients can receive some or all of their nutritional requirements via the gastrointestinal tract. Even when some component of nutritional support must be provided intravenously, feeding via the gut is desirable.
Provision of nutrition through the enteral route aids in prevention of gastrointestinal mucosal atrophy, thereby maintaining the integrity of the gastrointestinal mucosal barrier. Derangements in the barrier function of the gastrointestinal tract may permit the systemic absorption of gut-derived microbes and microbial products (bacterial translocation), which has been implicated as important in the pathophysiology of syndromes of sepsis and multiple organ system failure [13,14 and 15]. Other advantages of enteral nutrition are preservation of immunologic gut function and normal gut flora, improved use of nutrients, and reduced cost. Some studies suggest that clinical outcome is improved and infectious complications are decreased in patients who receive enteral nutrition compared with parenteral nutrition [16,17 and 18]. Additional clinical studies suggest that immune-enhancing enteral diets (immunonutrition) containing specialty nutrients (e.g., arginine, glutamine, nucleotides, and omega-3 fatty acids) may also reduce septic complications [19,20,21,22 and 23] but are not associated with an overall mortality advantage.
Several developments, including new techniques for placement of feeding tubes, availability of smaller caliber, minimally reactive tubes, and an increasing range of enteral formulas, have expanded the ability to provide enteral nutritional support to critically ill patients. Enteral feeding at a site proximal to the pylorus may be absolutely or relatively contraindicated in patients with increased risk of pulmonary aspiration, but feeding more distally (particularly distal to the ligament of Treitz) decreases the likelihood of aspiration. Other relative or absolute contraindications to enteral feeding include fistulas, intestinal obstruction, upper gastrointestinal hemorrhage, and severe inflammatory bowel disease. Enteral feeding is not recommended in patients with severe malabsorption or early in the course of severe short-gut syndrome.
Access to The Gastrointestinal Tract
After deciding to provide enteral nutrition, the clinician must decide whether to deliver the formula into the stomach, duodenum, or jejunum, and determine the optimal method for accessing the site, based on the function of the patient’s gastrointestinal tract, duration of enteral nutritional support required, and risk of pulmonary aspiration. Gastric feeding provides the most normal route for enteral nutrition, but it is commonly poorly tolerated in the critically ill patient because of gastric dysmotility with delayed emptying [24]. Enteral nutrition infusion into the duodenum or jejunum may decrease the incidence of aspiration because of the protection afforded by a competent pyloric sphincter; however, the risk of aspiration is not completely eliminated by feeding distal to the pylorus [25,26 and 27]. Infusion into the jejunum is associated with the lowest risk of pulmonary aspiration. An advantage of this site of administration is that enteral feeding can be initiated early in the postoperative period, because postoperative ileus primarily affects the colon and stomach and only rarely involves the small intestine. However, the early use of postpyloric feeding instead of gastric feeding in critically ill adult patients with no evidence of impaired gastric emptying was not associated with significant clinical benefits [28].
Techniques
Enteral feeding tubes can be placed via the transnasal, transoral, or percutaneous transgastric or transjejunal routes. If these procedures are contraindicated or unsuccessful, the tube may be
placed by endoscopy, using endoscopic and laparoscopic technique, or surgically via a laparotomy [29,30].
placed by endoscopy, using endoscopic and laparoscopic technique, or surgically via a laparotomy [29,30].
Nasoenteric Route
Nasoenteric tubes are the most commonly used means of providing enteral nutritional support in critically ill patients. This route is preferred for short-term to intermediate-term enteral support when eventual resumption of oral feeding is anticipated. It is possible to infuse enteral formulas into the stomach using a conventional 16 or 18 French (Fr) polyvinyl chloride nasogastric tube, but patients are usually much more comfortable if a small-diameter silicone or polyurethane feeding tube is used. Nasoenteric tubes vary in luminal diameter (6 to 14 Fr) and length, depending on the desired location of the distal orifice: Stomach, 30 to 36 inches; duodenum, 43 inches; jejunum, at least 48 inches. Some tubes have tungsten-weighted tips designed to facilitate passage into the duodenum via normal peristalsis; others have a stylet. Most are radiopaque. Newer tubes permit gastric decompression while delivering formula into the jejunum.
Nasoenteric feeding tubes should be placed with the patient in a semi-Fowler’s or sitting position. The tip of the tube should be lubricated, placed in the patient’s nose, and advanced to the posterior pharynx. If the patient is alert and can follow instructions, the patient should be permitted to sip water as the tube is slowly advanced into the stomach. To avoid unintentional airway placement and serious complications, position of the tube should be ascertained after it has been inserted to 30 cm. Acceptable means of documenting intraesophageal location of the tube include a chest radiograph or lack of co2 detection through the lumen of the tube by capnography or colorimetry. If the tube is in the airway, co2 will be detected and the tube must be removed. Proper final placement of the tube in the stomach must be confirmed by chest or upper abdominal radiograph before tube feeding is begun. The following methods to assess final tube placement are unreliable and do not assess tube misdirection into the lower respiratory tract: Auscultation over the left upper quadrant with air insufflation through the tube, assessment of pH with gastric content aspiration, and easy passage of the tube to its full length with the absence of gagging and coughing [31,32]. The tube should be securely taped to the nose, forehead, or cheek without tension.
Delayed gastric emptying has been confirmed in critically ill patients and may contribute to gastric feeding intolerance. One study randomized 80 critically ill patients to gastric feeding with erythromycin (200 mg intravenously [IV] every 8 hours as a prokinetic agent) or through a transpyloric feeding tube, and identified that the two were equivalent in achieving goal caloric requirements [33]. Spontaneous transpyloric passage of enteral feeding tubes in critically ill patients is commonly unsuccessful secondary to the preponderance of gastric atony. The addition of a tungsten weight to the end of enteral feeding tubes and the development of wire or metal stylets in enteral feeding tubes are aimed at improving the success rate for spontaneous transpyloric passage. Once the tube is documented to be in the stomach, various bedside techniques, including air insufflation, pH-assisted, magnet-guided [34], and spontaneous passage with or without motility agents may help to facilitate transpyloric feeding tube passage.
Intravenous metoclopramide and erythromycin have been recommended as prokinetic agents. But a Cochrane Database Systematic review concluded that doses of 10 or 20 mg of intravenous metoclopramide were equally ineffective in facilitating transpyloric feeding tube placement [35]. No matter which techniques are used to facilitate transpyloric passage of enteral feeding tubes, these tubes must be inserted by skilled practitioners using defined techniques [36,37].
If the tube does not pass into the duodenum on the first attempt, placement can be attempted under endoscopic assistance or fluoroscopic guidance. Endoscopic placement of nasoenteral feeding tubes is easily accomplished in the critically ill patient and can be performed at the bedside using portable equipment [38,39,40,41 and 42]. Transnasal or transoral endoscopy can be used for placement of nasoenteral feeding tubes in critically ill patients [42]. The patient is sedated appropriately (see Chapter 20), and topical anesthetic is applied to the posterior pharynx with lidocaine or benzocaine spray. A nasoenteric feeding tube 43 to 48 inches long with an inner wire stylet is passed transnasally into the stomach. The endoscope is inserted and advanced through the esophagus into the gastric lumen. An endoscopy forceps is passed through the biopsy channel of the endoscope and used to grasp the tip of the enteral feeding tube. The endoscope, along with the enteral feeding tube, is advanced distally into the duodenum as far as possible (Fig. 16-1).
The endoscopy forceps and feeding tube remain in position in the distal duodenum as the endoscope is withdrawn back into the gastric lumen. The endoscopy forceps are opened, the feeding tube released, and the endoscopy forceps withdrawn carefully back into the stomach. On first pass, the feeding tube is usually lodged in the second portion of the duodenum. The portion of the feeding tube that is redundant in the stomach is advanced slowly into the duodenum using the endoscopy forceps to achieve a final position distal to the ligament of Treitz (Fig. 16-2). An abdominal radiograph is obtained at the completion of the procedure to document the final position of the nasoenteral feeding tube. Endoscopic placement of postpyloric enteral feeding tubes is highly successful, eliminates the risk of transporting the patient to the radiology department for fluoroscopic placement, and allows prompt achievement of nutritional goals because enteral feeding can be initiated immediately after the procedure.
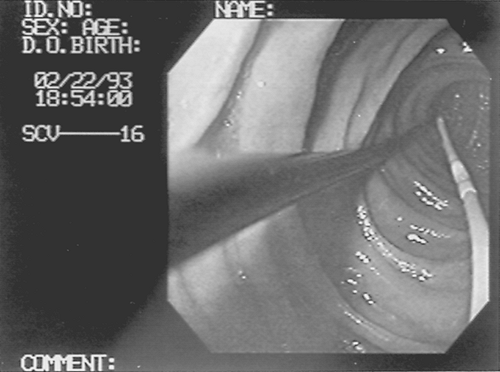 FIGURE 16-1. Endoscopic placement of nasoenteral feeding tube. Endoscopy forceps and gastroscope advance the feeding tube in the duodenum.

Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





