62 Endocarditis
• Endocarditis is an inflammation of the endothelial lining of the heart. It is usually focal and commonly occurs at points of endocardial injury. The mitral and aortic valves are the most common sites of involvement.
• Most sites of endocardial injury become seeded with bacteria during episodes of transient bacteremia and thus develop into infective endocarditis.
• The initial symptoms are often vague—low-grade fever, malaise, and weakness.
• Manifestations can vary from direct structural cardiac injury to conduction system disturbances, embolic phenomena, and cardiogenic or septic shock.
• Suspicion of infective endocarditis should be raised by the presence of well-known risk factors, such as acquired or congenital valvular or structural heart disease, a prosthetic valve, implanted medical devices, injection drug use, and a previous history of endocarditis.
• Laboratory testing is often not useful for the emergency physician, but at least three sets of blood cultures performed over time are critical for the diagnosis of infective endocarditis, as well as for guiding subsequent therapy.
• The most useful initial diagnostic test is echocardiography.
• In an acutely ill patient, prompt resuscitation, antibiotics, and surgical consultation are imperative.
• In a stable patient with subacute disease, time until initiation of antibiotic therapy is less critical than performance of serial blood cultures.
• Nearly all patients with infective endocarditis require hospital admission. Only the most stable patients with no complications in whom the diagnosis of infective endocarditis is being entertained but not confirmed may be discharged with very close follow-up care.
• Despite medical advances, the overall mortality for both native valve and prosthetic valve infective endocarditis still ranges from 20% to 25%.1
• Prevention of disease is most important. In 2007 the American Heart Association issued revised guidelines for antibiotic prophylaxis in patients at risk for endocarditis.
Epidemiology
Over the last 30 years, published reports regarding the overall incidence of IE have conflictingly cited both a stable incidence and a rising incidence.1–3 Mortality ranges from 5% to 50% or higher. The reason for such variation in the statistics is that IE is a diverse and evolving disease entity—one that is strongly influenced by the characteristics of both the human and microbial populations being studied (Table 62.1).
Table 62.1 Statistics for Infective Endocarditis (IE) in the Developed World
| Median age of IE patients in the preantibiotic era | 30-40 yr |
| Median age of IE patients in the antibiotic era | 47-69 yr |
| Mean male-to-female ratio | 1.7-2.0:1 |
| Incidence of community-acquired native valve IE (western Europe/United States) | 1.7-6.2 cases per 100,000 person-years |
| Incidence of IE in persons with known mitral valve prolapse | 100 cases per 100,000 person-years |
| Incidence of IE in injection drug users | 150-2000 cases per 100,000 person-years |
| Prosthetic valve IE | 7-25% of all cases of IE |
| Overall mortality for both native and prosthetic valve IE | 20-25% |
| Mortality with viridans group streptococci and Streptococcus bovis IE | 4-16% |
| Mortality with enterococci IE | 15-25% |
| Mortality with Q fever IE | 5-37% |
| Mortality with Staphylococcus aureus IE | 25-47% |
| Mortality with Pseudomonas aeruginosa, Enterobacteriaceae, or fungal IE | >50% |
Adapted from Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med 2001;345:1318-30.
In the developed world, IE has undergone a remarkable transformation over the last century. In the developing world, however, it has remained rather unchanged. Much of this difference is a result of the influence of advances in health care (e.g., antibiotics, disease prevention, medical devices, the resulting longevity of populations), as well as the complications that arise from these advances (e.g., nosocomial infections and resistant organisms).4,5
Unfortunately, the tremendous advances made in health care have not translated into the gains that we have seen in other infectious diseases in the last 50 to 80 years. Untreated, IE has a mortality of nearly 100%. When treated, however, IE is still associated with a mortality rate of 20% to 25%.5 The overall incidence of IE in the developed world has remained unchanged.1 Why has the advent of antibiotics, advanced critical care and surgical techniques, and medical devices such as prosthetic valves not made a difference in this statistic? There are several reasons.
First, with a low prevalence, no pathognomonic signs or symptoms, and no single diagnostic front-line test, IE remains difficult to diagnose. Therefore, many cases are missed or diagnosed only when the disease is advanced. Second, despite the effective control of rheumatic heart disease in the developed world, new risk factors have arisen to fill the void. Degenerative heart disease in the growing elderly population has replaced rheumatic fever as the major cause of valvular disease. The same intravascular medical devices that have improved survival for patients (e.g., valvular prosthetics, cardiac pacemakers, long-term indwelling vascular catheters) predispose them to the development of IE (regardless of whether they have had IE in the past). Third, the number of patients at risk for IE has increased—the elderly, patients receiving critical care, and immunocompromised patients (because of acquired immunodeficiency syndrome, diabetes mellitus, end-stage renal disease, chemotherapy, and other reasons). Risky social behavior, such as body piercing and injection drug use, is practiced more today than in the early 20th century. Finally and most concerning of all, burgeoning antibiotic resistance is making treatment of IE more challenging and sometimes unachievable.6
Pathophysiology
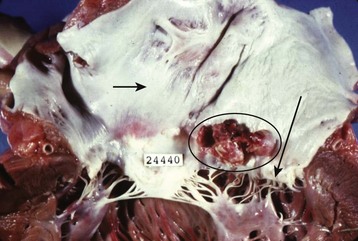
The term endocarditis literally means inflammation of the inner lining or endothelium of the heart or lining of heart valves (or both). Local or systemic stressors, such as trauma, blood-borne contaminants (e.g., talc from injection drug use), inflammation, and abnormal blood turbulence, induce injury to the endothelium. Clinically relevant endocarditis results from the formation of a fibrin and platelet cap on the area of altered surface endothelium. Most commonly, a sterile cap forms at a site of endothelial injury. IE occurs when microbes adhere to these sites of sterile endothelial injury during transient periods of bacteremia, fungemia, or viremia. Colonization occurs, followed by microbial multiplication and growth of each cap into a vegetation (Figs. 62.1 and 62.2). Because of their direct contact with the bloodstream, these infections cause a continuous, albeit low-level presence of microbes in the blood. The clinical manifestations of endocarditis are quite varied as a result of immunologic, infectious, and embolic processes. It is this variation in manifestations that often makes endocarditis difficult to identify.
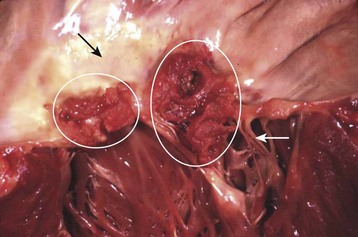
Fig. 62.1 Large vegetations (circles) at the edge of this mitral valve (black arrow).
Chordae tendineae (white arrow) connect the mitral valve to papillary muscles in the left ventricle.
(Courtesy Charles C. Marboe, MD.)
Microbiology of Infective Endocarditis
Although the microbiology of IE can predict the course of a patient’s illness and guide therapy, the actual infecting organism is rarely known to the EP. The EP needs to know the microbes that cause IE (Box 62.1) and the local resistance patterns to make sound choices regarding empiric antibiotic treatment regimens. This section discusses the organisms most commonly associated with IE.6 Certain patient characteristics and clinical scenarios are associated with particular microorganisms (Table 62.2). These scenarios may guide the EP’s choice of empiric antibiotics; specific regimens are discussed later in this chapter (see Table 62.4).
Box 62.1
Microorganisms that Cause Infective Endocarditis (Approximate Percentage)
Adapted from Baddour LM, Wilson WR, Bayer AS, et al, for the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; Council on Cardiovascular Disease in the Young; Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia; American Heart Association; Infectious Diseases Society of America. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications. A statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005;111:e394-434; and Murdoch DR, Corey GR, Hoen B, et al, for the International Collaboration on Endocarditis—Prospective Cohort Study (ICE-PCS) Investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century. Arch Intern Med 2009;169:463-73.
Table 62.2 Characteristics of Patients with Infectious Endocarditis and Associated Microorganisms
| CHARACTERISTIC | ORGANISM | COURSE/FACTS* |
|---|---|---|
| Community-acquired IE involving a native valve | Viridans group streptococci | |
| Staphylococcus aureus | ||
| Prosthetic valve IE < 1 mo after surgery | Staphylococcus epidermidis | |
| Prosthetic valve IE > 1 mo after surgery | S. aureus | |
| Elderly patient | Enterococci | |
| Elderly patient with a GI process | Streptococcus bovis | |
| Injection drug user | S. aureus | |
| Viridans group streptococci | ||
| Pseudomonas aeruginosa | ||
| Fungi | ||
| Patient is critically ill, is being treated in an intensive care unit, or is immunocompromised | Fungi | |
| P. aeruginosa |
GI, Gastrointestinal; IE, infective endocarditis.
* Data from Baddour LM, Wilson WR, Bayer AS, et al, for the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; Council on Cardiovascular Disease in the Young; Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia; American Heart Association; Infectious Diseases Society of America. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications. A statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005;111:e394-434.
Bacteria
Viridans Group Streptococci
Streptococcus viridans, formerly a species name, is actually a group of gram-positive cocci. This group has been the most common cause of IE, although more recent case series have shown that Staphylococcus aureus may now be more common.6,7 These streptococci usually seed previously damaged cardiac tissue. The clinical findings are usually more insidious, however, with patients experiencing malaise, weakness, and low-grade fever.
Staphylococcus aureus
Studies have now identified S. aureus rather than the viridans group of streptococci as the most common cause of IE.1,7 S. aureus can infect normal valvular endothelium—that is, endothelium without antecedent damage or disease—and usually causes aggressive valve destruction. It is associated with injection drug use, as well as with prosthetic valve endocarditis that occurs more than 1 month after surgery.
Over the last decade, the story of S. aureus and S. aureus IE has become increasingly complicated with the emergence of methicillin-resistant S. aureus (MRSA), as well as the subsequent identification of community-associated (CA-MRSA) and hospital-associated (HA-MRSA) subtypes. CA-MRSA has a tendency to affect previously healthy individuals but has a drug sensitivity pattern more favorable than that of HA-MRSA. HA-MRSA tends to affect the infirm (hospitalized, nursing home, elderly, preterm, and immunocompromised patients) and has a limited sensitivity pattern. A review of cases of native valve IE caused by these organisms reveals a higher mortality rate with HA-MRSA (37%) than with methicillin-sensitive S. aureus and CA-MRSA (23% and 13%, respectively).8
Culture-Negative Bacteria
The culture-negative bacteria group infrequently causes IE. These bacteria are characterized as culture negative because they either grow slowly in routine media, require special media to grow, or cannot be cultured. If clinical suspicion exists, the clinician must ask that blood cultures be held for a prolonged incubation period (14 to 21 days), request special culture media, or use the serologic and polymerase chain reaction assays available for some of these bacteria. A list of culture-negative bacteria is provided in Box 62.1.
Fungi
Fungi are rarely a cause of endocarditis, but fungal IE has high mortality. Candida species are responsible for most cases of fungal IE. Aspergillus species are also seen. Fungal IE tends to occur in patients with cardiac abnormalities, medical devices (prosthetic valves, long-term indwelling vascular catheters), some level of compromised immunity (human immunodeficiency virus, malignancy, organ transplantation), and injection drug use.9 Fungal IE usually produces large vegetations and is an indication for surgical intervention.
Presenting Signs and Symptoms
IE can vary greatly in the severity of its manifestations. Depending on the extent of the injury, location of the injury, microorganism involved, and comorbid conditions in the patient, IE can be an insidious chronic or subacute disease or an aggressive, rapidly debilitating process. Recent prospective cohort data from an international multicenter study have revealed that the acute manifestation is becoming more common—perhaps because of the increasing prevalence of S. aureus IE.7
EPs must maintain high clinical suspicion in situations associated with IE. Patients at high risk for IE are listed in Box 62.2. In such patients, sepsis, embolization, or cardiac failure or shock should warrant an evaluation for endocarditis. By understanding the pathophysiology of this disease, the clinician can predict the signs and symptoms that might be seen with IE.
Box 62.2
Risk Factors for Infective Endocarditis
Acquired or congenital valvular and structural heart disease, including mitral valve prolapse, rheumatic heart disease, and hypertrophic cardiomyopathy
Prosthetic valves, including bioprosthetic devices
Implantable medical devices (cardiac pacemakers, long-term indwelling vascular catheters, implantable defibrillators)
Previous history of endocarditis
Adapted from Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med 2001;345:1318-30.
Classic Triad
The triad consisting of fever, heart murmur, and anemia has classically been ascribed to the diagnosis of IE. Unfortunately, the sensitivity and specificity of these findings for endocarditis are poor. The clinician must combine these findings with high-risk patient characteristics (see Box 62.2).
Organ-Specific Clinical Findings
Vascular Signs and Symptoms
The signs and symptoms that may be seen with involvement of specific vascular sites are as follows:
• Central nervous system (CNS) arteries—headache, focal neurologic deficits, confusion
• Sinus of Valsalva—pleuritic chest pain, muffled heart tones
• Hepatic artery—right upper quadrant pain, hematemesis
• Splenic artery—abdominal pain, intraabdominal hemorrhage
• Renal arteries—flank pain, hematuria
• Intestinal arteries—abdominal pain, intraabdominal hemorrhage, melena, hematochezia.