Head Injury and Neuroanesthesia
Air elimination capability in rapid infusion systems
Zoremba N, Gruenewald C, Zoremba M, et al (Univ Hosp RWTH Aachen, Germany; Med Ctr Marienhöhe Würselen, Germany; Univ Hosp Marburg, Germany) Anaesthesia 66:1031-1034, 2011§
Evidence Ranking
• B
Expert Rating
• 2
Abstract
Pressure infusion devices are used in clinical practice to apply large volumes of fluid over a short period of time. Although air infusion is a major complication, they have limited capability to detect and remove air during pressure infusion. In this investigation, we tested the air elimination capabilities of the Fluido® (The Surgical Company), Level 1® (Level 1 Technologies Inc.) and Ranger® (Augustine Medical GmbH) pressure infusion devices. Measurements were undertaken with a crystalloid solution during an infusion flow of 100, 200, 400 and 800 ml.min−1. Four different volumes of air (25, 50, 100 and 200 ml) were injected as boluses in one experimental setting, or infused continuously over the time needed to perfuse 2 l saline in the other setting. The perfusion fluid was collected in an airtight infusion bag and the amount of air obtained in the bag was measured. The delivered air volume was negligible and would not cause any significant air embolism in all experiments. In our experimental setting, we found, during high flow, an increased amount of uneliminated air in all used devices compared with lower perfusion flows. All tested devices had a good air elimination capability. The use of ultrasonic air detection coupled with an automatic shutoff is a significant safety improvement and can reliably prevent accidental air embolism at rapid flows (Fig 1, Table 1).

Full access? Get Clinical Tree


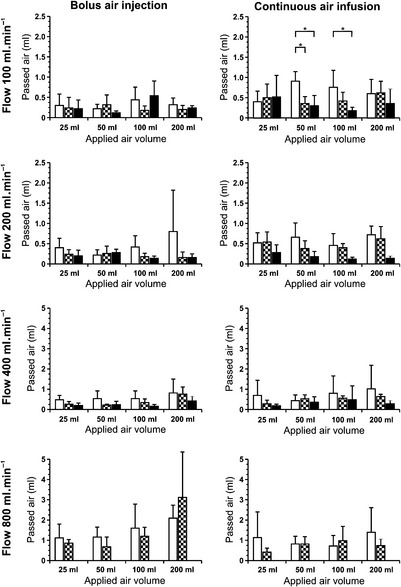
 ), Level 1 (
), Level 1 ( ) and Ranger (
) and Ranger ( ) pressure infusion devices at different flow rates, during bolus (left) and continuous (right) injection of air. (In the Ranger, a flow of 800 ml.min−1 was not tested because this exceeded the maximum infusion flow of 500 ml.min−1). Error bars represent SD. *p<0.05. (Reprinted from Zoremba N, Gruenewald C, Zoremba M, et al. Air elimination capability in rapid infusion systems. Anaesthesia. 2011;66:1031-1034, with permission from The Authors.)
) pressure infusion devices at different flow rates, during bolus (left) and continuous (right) injection of air. (In the Ranger, a flow of 800 ml.min−1 was not tested because this exceeded the maximum infusion flow of 500 ml.min−1). Error bars represent SD. *p<0.05. (Reprinted from Zoremba N, Gruenewald C, Zoremba M, et al. Air elimination capability in rapid infusion systems. Anaesthesia. 2011;66:1031-1034, with permission from The Authors.)
