Electrical Therapies
Peter J. Kudenchuk
A good rule of thumb for determining when to use synchronized versus unsynchronized shock is that if the provider cannot identify each QRS complex with the eye, then the defibrillator will likely not be able to identify them either. In these instances, unsynchronized shock at defibrillation energy settings should be administered.
Synchronized and unsynchronized shocks
Indications for treating emergency pacing in emergency cardiovascular care (ECC)
Special considerations for treating patients with pacemakers and implantable cardioverter defibrillators (ICDs)
Management of bradyarrhythmia and conduction disturbances in acute myocardial infarction (AMI)
Safety considerations using electrical therapy
Cardioversion and Defibrillation1
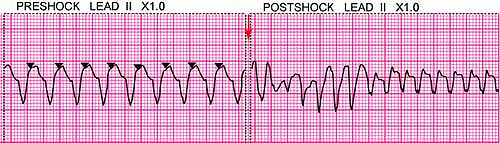
Shock therapy (cardioversion or defibrillation) is recommended for any arrhythmia caused by reentry that requires termination. This includes atrioventricular nodal reentry tachycardia, atrioventricular reentry tachycardia, intra-atrial reentry tachycardia, sinus node reentry tachycardia, atrial fibrillation, atrial flutter, ventricular tachycardia, and ventricular fibrillation (see also Chapter 22). These arrhythmias are caused by reentry (an abnormal rhythm circuit that is sustained by circulating upon itself again and again), which can be interrupted by a shock that breaks the circulating pattern (Fig. 24-1).
Shock therapy will not be effective for treatment of automatic tachycardias such as ectopic atrial tachycardia, multifocal atrial tachycardia or junctional tachycardia. Such automatic rhythms are created when local cells become more excited and spontaneously depolarize (fire) more rapidly. Sinus tachycardia is a good example of an automatic rhythm. It results when cells in the sinus node are excited to fire more rapidly. Junctional tachycardia, or ectopic or multifocal atrial tachycardia, is created by the same mechanism but at a different location. An understanding of this mechanism will help to clarify why automatic rhythms are not responsive to shock. Shock stops reentry rhythms by breaking their circulating pattern. Shock cannot stop a rhythm that originates from locally excited cells. In fact, shocking any automatic rhythm is no different than shocking sinus tachycardia—it only results in more of the same. In fact, shock delivery to tachy-arrhythmias caused by a rapid automatic focus may actually increase the rate of the tachyarrhythmia because of the enhanced sympathetic tone resulting from shock.
Synchronized Electrical Shock (Cardioversion)
Synchronized cardioversion is recommended for any arrhythmia that is caused by reentry, has distinguishable QRS complexes to which a shock could be synchronized, and that needs to be terminated because of the patient’s clinical condition. Synchronized electrical shock (cardioversion) refers to the administration of an electrical shock that is timed to occur simultaneously with the QRS complex. Synchronized cardioversion permits the use of lower-energy settings to convert an organized rhythm, and synchronizing the shock during the QRS complex minimizes the risk of delivering the shock on the T wave, which could induce ventricular fibrillation (VF).
Synchronization is an automated feature of defibrillators that senses QRS complexes based on their electrical characteristics in the lead under evaluation. While mostly reliable, it is not foolproof and on occasion may inappropriately synchronize to another portion of the ECG, such as the T wave, with disastrous consequences. Thus, it is critical for the responder to confirm that synchronization is appropriately timed before shock delivery. This is done by seeing whether the synchronization markers on the defibrillator’s ECG screen appropriately correspond to where QRS complexes occur (Fig. 24-2). If the timing is incorrect in the lead, a different ECG lead should be selected where synchronization is reliably timed to the QRS. Done properly, the risk of cardioversion is relatively small.2
Unsynchronized Shock (Defibrillation)
For some arrhythmias, synchronization is not possible or recommended (Fig. 24-1). Synchronization requires that the defibrillator be able to reliably recognize a consistent QRS to which a shock can be precisely timed. If the defibrillator is unable to synchronize, it will withhold delivery of any shock. The many QRS configurations and irregular rates comprised by polymorphic ventricular tachycardia and VF may make it difficult or impossible for the defibrillator to reliably synchronize to a QRS complex. Likewise, it may not be possible for a defibrillator to synchronize to a monomorphic ventricular tachycardia that is extremely rapid in rate. This can result in delay of an emergently needed shock. For this reason unsynchronized shock (defibrillation) is recommended in all pulseless states, as for VF and pulseless ventricular tachycardia (VT), where any delay in shock treatment cannot be afforded.
A good rule of thumb for determining when to use synchronized versus unsynchronized shock is that if the provider cannot identify each QRS complex with the eye, then the defibrillator will likely not be able to identify them either. In these instances, unsynchronized shock at defibrillation energy settings should be administered.
A second important rule is that maximum energy settings should be used for any unsynchronized shock. Lower energy settings should not be used for unsynchronized shocks because they have a higher likelihood of provoking VF when given in an unsynchronized fashion.
So-called precordial thump cardioversion, in which the midchest is struck by the fist, should be considered equivalent to unsynchronized, very low energy cardioversion and has been proven ineffective; therefore, it is not a recommended intervention for ventricular tacharrhythmias.3
Defibrillation Electrode Position
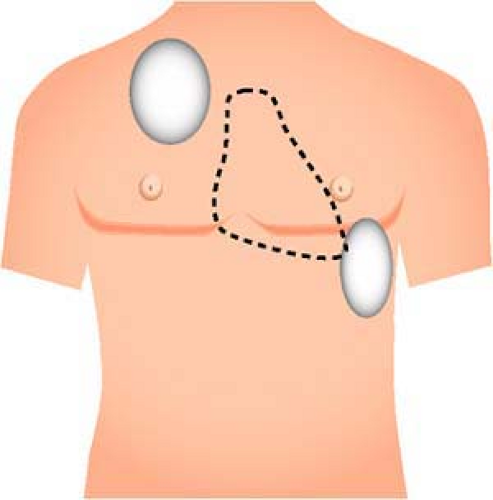
For ventricular defibrillation, conventional right anterior (infraclavicular) and left lateral (apical) defibrillation electrode locations continue to be recommended because of the ease of placement and the efficacy. The anterior electrode should be placed just below the right clavicle, with the center of its electrode at the midclavicular line; the apical electrode should be placed at the inferolateral left chest, left of and just below the nipple (lateral to the left breast in women), with the center of its electrode in the midaxillary line (Fig. 24-3).
Mistakes in Pad Position or Size
One of the most common errors in pad placement is to place the pads too close together. Figure 24-4A displays a cross section of the heart and thorax showing how most of the current bypasses the heart when the paddles are placed too close together. Notice also that the sternal paddle is indeed over the sternum, which blocks much of the current flow. In Figure 24-4B, the apex paddle is in the proper position in the midaxillary line, which allows all the current to flow through the myocardium, achieving defibrillation.
An interesting manikin study from England supports the idea that improper placement of the apex pad or paddle is the most common positional error.5 During a course on resuscitation techniques, doctors were asked to place the two defibrillator paddles in the “proper” locations on the breast plate of a resuscitation training manikin. Figure 24-5 presents a composite “map” of all the locations selected by the 101 subjects. This map unequivocally shows how the doctors erred in paddle placement more than 90% of the time, placing the apex paddle too far anterior, well away from the recommended location near the axillary line. Look again at Figure 24-4B to see how, somewhat counterintuitively, moving the apex paddle away from the heart and toward the axillary line will lead to more effective defibrillatory shocks.
For cardioversion, particularly of atrial fibrillation, other electrode positions have been suggested, including placement directly opposite each other in an anteroposterior configuration over the mid- or left-chest wall and back or at the right anterior mid-chest wall and the left lower scapular region.6,7 However, direct comparison of these configurations has not demonstrated a significant difference in shock success.8 On occasion, electrode positions need to be modified because of patient anatomy, location of an implanted pacemaker or defibrillator (see below), or other extenuating circumstances. Alternate positions may include placement of electrodes on the right and left lateral chest wall (biaxillary) or the left electrode in the standard apical position and the other electrode on the right or left upper back. Whatever defibrillation electrode positions are ultimately selected, conceptually the idea is to “sandwich” the heart between the electrodes to ensure maximum coverage by the shock.
Shock Waveform
Shock waveforms for external cardioversion and defibrillation vary by device manufacturer. The shock waveforms traditionally used, consisted of a single-phase shock (either a damped sinusoidal monophasic or truncated exponential monophasic waveform). These have been largely replaced by biphasic waveform defibrillators, which administer a two-phase shock with each phase being of opposite polarity. Biphasic defibrillators administer differing forms of biphasic waveform shock (such as biphasic truncated exponential and rectilinear biphasic) and have different energy setting options depending upon the manufacturer. Evidence suggests that biphasic waveform shock is more effective than monophasic shock for cardioversion of atrial fibrillation10,11 and may be as good or better for defibrillation of VF and pulseless VT.12,13
Energy Settings: Supraventricular Tachycardia
The recommended initial shock energy setting for cardioversion of atrial fibrillation with a monophasic waveform should be no less than 100 J. Typically a 200-J energy setting is used. Cardioversion of atrial flutter and paroxysmal supraventricular tachycardia (PSVT) may require less energy (25–50 J).14 Current AHA ECC guidelines recommend starting with a dose of 50 to 100 J and proceeding in a stepwise fashion if the initial shock does not convert the rhythm.15 However, factors such as patient’s clinical condition, use of medications, and transthoracic resistance are important determinants of the energy required for successful cardioversion. From the standpoint of patient comfort, there is little advantage to using lower energy settings. Lower energy settings also risk the need for repeated shock if not initially successful and can precipitate VF if not appropriately timed for delivery precisely on the QRS. For these reasons some experts begin with energy settings of 200 J for virtually all supraventricular arrhythmias, and, if unsuccessful, increasing the setting in a stepwise fashion up to the maximum of 360 J.16
Cardioversion with biphasic waveforms is now available,17 but more data are needed before specific comparative dosing recommendations can be made. In general, for a given shock energy setting, biphasic waveform shock is more likely to terminate atrial arrhythmias than monophasic waveform shock, and biphasic waveform shock can be deployed at a lower energy setting than monophasic shock, with comparable success.18,19 For example, an initial biphasic shock setting as low as 70 J successfully terminated atrial fibrillation more effectively than a 100-J monophasic waveform shock.18
Energy Settings: Ventricular Arrhythmias
Monomorphic VT, defined as having QRS complexes of uniform appearance and occurring at a regular rate, responds to monophasic or biphasic synchronized cardioversion at relatively low energy settings of 25 to 50 J; if there is great urgency to terminate VT, one can begin with energy settings of 100 J or higher.20 Less organized ventricular arrhythmias, such as polymorphic VT and VF, typically require higher energy settings, which should be administered in unsynchronized fashion. If a monophasic defibrillator is used, 200 to 360 J has generally been used, but current AHA guidelines recommend that 360 J be used for all shocks.
The recommended initial shock energy setting with a biphasic defibrillator is 150 to 200 J; an equal or higher dose is recommended for second and subsequent shocks. Whether the success of defibrillation in out-of-hospital cardiac arrest is improved by using monophasic or biphasic waveform shock is controversial,12,21 as is whether higher biphasic waveform shock settings (360 J) are more beneficial than lower settings (200 J) during resuscitation of a cardiac arrest patient.22 After shock delivery, the health care provider should be prepared to provide immediate cardiopulmonary resuscitation (CPR) and other needed support.
Pacing
Emergency pacing of the heart has been greatly simplified by the development of devices able to apply a pacing stimulus through the chest wall. The technique of using these devices is called transcutaneous pacing (TCP). In an emergency setting, TCP can be initiated rapidly and noninvasively, obviating the time to prepare for transvenous pacing and the complications associated with emergency access of the central circulation. Historically, emergency pacing has been employed both in and out of hospital during cardiac arrest and for patients with serious symptoms or hemodynamic instability due to bradycardia.
Other forms of pacing include standby (or “backup”) pacing, provided primarily when clinical or electrocardiographic findings indicate the likelihood of progression to high-degree block but pacing is not immediately required. Temporary pacing is often used as a bridge to permanent pacing when a correctable condition cannot be reversed or cardiac pathology causing block is anticipated to be fixed or to transiently compromise function. Emergency health care providers are not usually involved in decisions for permanent pacemaker implantation but should be aware of conditions likely to necessitate continued need for pacemaker therapy. Early involvement of a cardiology or electrophysiology expert is often important in staging devices and procedures.
Emergency Pacing
TCP is an indicated intervention for symptomatic bradycardias. It should be started immediately for patients who are unstable, particularly those with high-degree (Mobitz type II second-degree or third-degree) block. TCP can be painful, and some limitations apply. Pacing spikes may be present, but electrical stimulation may fail to produce effective mechanical capture. After starting pacing, carefully assess the patient for clinical response. Because heart rate is a major determinant of myocardial oxygen consumption, set the pacing rate to the lowest effective rate based on clinical assessment and symptom resolution. If cardiovascular symptoms are not caused by the bradycardia, the patient may not improve despite effective pacing.
Standby Pacing
The indications for standby pacing are multiple and most often occur in the setting of an acute coronary syndrome (ACS). These patients typically are clinically stable yet at risk for decompensation in the near future. Often it is the location and size of MI that is ominous in these patients. The development of the rhythm disorder is a secondary event, and pacing has not altered outcome in the past. Priority is given to reperfusion of patients with STEMI, and treatment of symptomatic AV block is supportive in the vast majority of patients.
Because TCP is widely available and inexpensive, when advanced cardiac life support (ACLS) providers are concerned about the possibility of the development of high-degree symptomatic AV block, preparations for TCP should be initiated. Standby TCP has also been used successfully during surgery for high-risk patients who have bifascicular or left bundle-branch block with additional first-degree block.23,24 If it is then needed to treat hemodynamically significant bradycardia, the device provides a therapeutic bridge until a transvenous pacemaker can be placed under more controlled circumstances. Although it is more invasive, a transcutaneous pacemaker can also be initially placed in standby mode for these and other at-risk patients.
Pacing for Pulseless Bradyasystolic Cardiac Arrest
Pacing has been studied extensively in the treatment of pulseless patients with bradycardia or asystole. Some studies had shown encouraging results in such patients when pacing was initiated within 10 minutes of cardiac arrest, but recent studies have documented no improvement in either short-term outcomes (admission to hospital) or intermediate-term outcomes (survival to hospital discharge).25,26,27
Prehospital studies of TCP for asystolic arrest or postshock asystole have also shown no benefit of pacing.28 In a level 1, prospective, controlled trial of TCP for cardiac arrest, investigators observed no benefit even when CPR was combined with pacing, nor did they observe any benefit when the asystole was of only brief duration after a defibrillatory shock.27
Pacing for Drug-Induced Cardiac Arrest
An exception to the negative results of pacing for cardiac arrest involves patients in cardiac arrest provoked by overdose of cardiac medications such as calcium channel blockers or anti-arrhythmic agents. In such cases, pacing may be successful for the treatment of profound bradycardia or pulseless electrical activity.29,30,31,32,33 Emergency pacing may also benefit patients with pulseless electrical activity due to acidosis or electrolyte abnormalities. Such patients often possess a normal myocardium with only temporary impairment of the conduction system.
While attempts are made to correct electrolyte abnormalities or profound acidosis, pacing can stimulate effective myocardial contractions. Similarly, pacing can be life-sustaining as the conduction system recovers from the cardiotoxic effects of a drug overdose or poisoning with other substances.32
Temporary Pacing
Although TCP is a recommended intervention for symptomatic bradycardia, it can be painful, without complete assurance of capture, and should serve only as an interim measure until more reliable transvenous pacing can be accomplished. TCP is generally ineffective in patients with cardiac arrest due to asystole or pulseless electrical activity,27,34 perhaps with the exception of where associated with drug overdose.29,30,31,32,33
TCP is performed from the same defibrillator electrodes used for cardioversion and defibrillation. However, anteroposterior positioning of the electrodes between the chest and back is recommended in order to capture ventricular muscle. Typically, a left anterior parasternal and left posterior paraspinal (directly opposite the anterior electrode) or left anterior parsternal and right posterior paraspinal position is deployed. If one electrode configuration does not result in ventricular capture, other configurations can be attempted. However, not all patients can be successfully paced with TCP. Such pacing results also in local
muscle stimulation and will be painful to the awake patient. It should only serve as a bridge to transvenous temporary pacing if required. Notably, the same electrodes used for pacing may be required for defibrillation (and vice versa). Fortunately, the positions used for TCP usually accommodate their use for defibrillation. However, the care provider may need to prioritize the immediate need of the patient (pacing versus defibrillation) in selecting the location where electrodes are initially placed and be prepared to relocate them if necessary. Output from the pacemaker should be increased from the minimal setting until a pacing artifact appears on the monitor screen, followed by a widened QRS complex and T wave, which indicates ventricular capture. Ventricular capture is not completely reliable, and it can be challenging to determine such capture from the rhythm monitor because of the degree of electrical artifact created by TCP. One should specifically evaluate for the presence of a pacer spike, followed by a QRS complex, followed by a repolarization (T) wave, and confirmed by the presence of a palpable pulse, aterial pressure tracing, or measured blood pressure (Fig. 24-6).35
muscle stimulation and will be painful to the awake patient. It should only serve as a bridge to transvenous temporary pacing if required. Notably, the same electrodes used for pacing may be required for defibrillation (and vice versa). Fortunately, the positions used for TCP usually accommodate their use for defibrillation. However, the care provider may need to prioritize the immediate need of the patient (pacing versus defibrillation) in selecting the location where electrodes are initially placed and be prepared to relocate them if necessary. Output from the pacemaker should be increased from the minimal setting until a pacing artifact appears on the monitor screen, followed by a widened QRS complex and T wave, which indicates ventricular capture. Ventricular capture is not completely reliable, and it can be challenging to determine such capture from the rhythm monitor because of the degree of electrical artifact created by TCP. One should specifically evaluate for the presence of a pacer spike, followed by a QRS complex, followed by a repolarization (T) wave, and confirmed by the presence of a palpable pulse, aterial pressure tracing, or measured blood pressure (Fig. 24-6).35
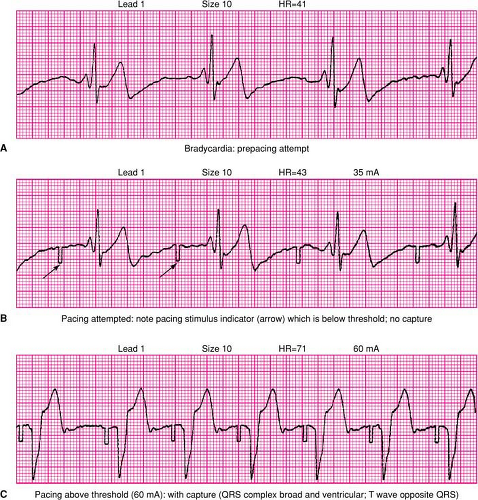 Figure 24-6 • Transcutaneous pacing. A. Sinus bradycardia is shown without evidence of pacing stimulation. B. Transcutaneous pacing is activated, evidenced by the presence of pacemaker artifact (arrow) but without capture of the ventricle. C.
Get Clinical Tree app for offline access

Full access? Get Clinical Tree


|