Chapter 23 Echocardiography and Noninvasive Diagnosis
The technique of echocardiography and the practice of “echocardiology” have changed the practice of pediatric cardiology by largely replacing cardiac catheterization/angiography for the diagnosis of congenital malformations. Combined with use of prostaglandin for maintaining the patency of the ductus arteriosus, echocardiography has dramatically reduced the need for emergency cardiac catheterization in neonates. Most patients with congenital heart disease detected in the neonatal period can undergo palliative surgery without cardiac catheterization. Most definitive surgical repairs can be performed successfully without the risk of invasive studies. Pulsed, continuous-wave, color, and tissue Doppler have added important capabilities for anatomic and functional assessment. Intraoperative and postoperative management of congenital heart defects has been aided by the addition of transesophageal echocardiography (TEE). This mode can improve resolution in neonates and older patients in whom transthoracic imaging is difficult, and greatly aids the surgeon by providing immediate feedback about the quality of the repair prior to separation from the heart-lung machine. Higher-resolution imaging systems continue to evolve. Multielement transducer technology and advances in high-speed computing, three-dimensional real-time imaging, color Doppler, and tissue Doppler have facilitated the assessment of systolic and diastolic function of the myocardium.1 This chapter focuses on the detection of congenital heart disease in pediatric patients presenting with cardiopulmonary compromise at any age and illustrates the use of TEE for physiologic assessment and management.
Diagnosis of Congenital Heart Disease
The segmental approach is based on the principle that all aspects of abnormal cardiovascular morphology can be broken down into discrete, mutually exclusive descriptors, allowing unambiguous delineation of any complex congenital malformation. The schema must include information on the presence, position, and connection of each cardiac segment. Classically, three segments have been recognized: atria, ventricles, and great arteries. By describing the anatomic segments and indicating the normality or abnormality of each, a complete description of the cardiac anatomy is possible. It now is possible to code cardiac anatomic abnormalities by segmental analysis.2
Cardiac Function Assessment
Doppler echocardiography can provide functional information that is not available by any other method. Pulsed Doppler can interrogate a site in the circulation and measure the direction and speed of blood flow in systole and diastole. For example, sampling in the aorta allows comparison of upper and lower body resistances to blood flow by revealing the direction of flow in systole and diastole. The structure and function of the cardiac valves can be determined, perhaps the most powerful application of echocardiography. For example, atrioventricular (AV) valve regurgitation can be diagnosed and its severity, which depends on many technical and physiologic factors, can be estimated. In addition to visualizing a ventricular septal defect (VSD), the jet of a left-to-right shunt can be detected by pulsed Doppler, with the pressure gradient quantified by continuous-wave Doppler (using the simplified Bernoulli equation: Pressure gradient = 4V2, where V is peak velocity), and the defect spatially localized by color Doppler. Using the continuity equation and the proximal isovelocity surface area (PISA) concept, flow area and volume can be calculated in left-to-right shunts, and the regurgitant volume and area can be calculated in AV valve regurgitation. Pulmonary artery pressure often can be estimated using the peak velocity of the tricuspid regurgitation jet, and the severity of semilunar stenosis or coarctation can be estimated using the peak and mean gradients. This hemodynamic information can be integrated into the segmental description using the anatomic segment as the finding and the functional aspect as the modifier. For example, the morphologic mitral valve is an anatomic site and location, for which regurgitation might be a modifier. This step-by-step approach to diagnosis answers specific questions about cardiac function and screens for congenital and acquired abnormalities.3,4
Structure-Oriented Approach
Adult-oriented, two-dimensional echocardiographic reports usually are based on standard views of the cardiac anatomy that are highly reproducible. The investigators describe the appearance of a given cardiac lesion as seen on a standard parasternal, apical, or subcostal scan. However, this approach can lead to diagnostic errors when applied to congenital heart disease.5 For example, a scan of an aortopulmonary window from the right ventricular outflow tract can simulate the origin of the aorta from the right ventricle (i.e., transposition of the great arteries). In congenital heart disease, the various views must be integrated, scanning from one echocardiographic window to another to obtain a complete anatomic examination. Although the echocardiographic examiner with experience learns to identify the normal appearances of the heart without congenital defects from various echocardiographic windows, a structure-oriented or anatomic approach always is superior to an approach based on standardized views.
Achieving high sensitivity in the detection of congenital heart disease requires a compulsive approach in order to locate rare anatomic variations that may be important. For example, coronary artery anomalies, such as a coronary originating from the pulmonary artery, can be reliably detected using a standardized approach to defining the origins and courses of the coronary branches.6
Segmental Analysis: Situs Diagnosis
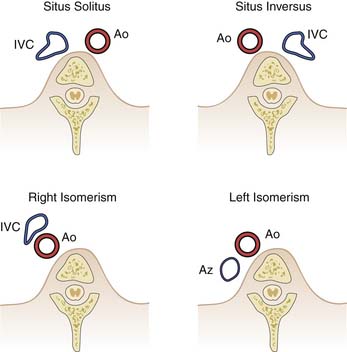
Determination of cardiac position and atrial-visceral situs is a standard portion of the echocardiographic assessment of congenital heart disease and is the foundation of the segmental approach. Atrial situs and atrial morphology are diagnosed together, and four possibilities exist: solitus (normal), inversus, and heterotaxy that may be right atrial isomerism or left atrial isomerism. For example, for situs solitus, the morphologic right atrium is on the right and the morphologic left atrium is on the left. Abnormal atrial situs and cardiac malposition, such as dextrocardia, frequently are associated. Both can be diagnosed by obtaining a short-axis scan of the abdomen, identifying the spine and the inferior vena cava and the descending aorta. The location of the cardiac apex is important for later scanning from the apex. Subcostal scanning above the diaphragm immediately shows the position of the cardiac apex. From this scan below the diaphragm, the position of the inferior vena cava and aorta can usually be identified, and their location with respect to the spine identifies the situs (Figure 23-1).
The descending aorta and inferior vena cava are oriented symmetrically with respect to one another, with the inferior vena cava to the right in situs solitus and to the left in situs inversus. In right atrial isomerism, the aorta and inferior cava run together on either side of the spine, with the cava anterior. A venous structure that courses behind the aorta and does not enter the heart suggests azygos continuation of the inferior vena cava, which is associated with left atrial isomerism. These patients usually have separate, anomalous hepatic venous connections to the heart. Occasionally, atrial appendage morphology can be identified and the diagnosis of atrial situs confirmed directly. A broad-based atrial appendage usually is a morphologic right one, and a narrow-based appendage is a morphologic left one. Symmetrical appendages suggest atrial isomerism. Situs diagnosis is important clinically for the care of the intensive care patient. Complex congenital malformations occur predictably with right and left isomerism, and asplenia (associated with right atrial isomerism) may place the child at risk for recurrent or persistent infection. Left isomerism is associated with a high rate of gastrointestinal obstruction after birth.
Segmental Analysis: Atrioventricular Connection
Description of the connection of the atria and ventricles (i.e., AV connection) requires knowledge of both atrial and ventricular morphology. The echocardiographic criteria for a morphologic left ventricle include insertion of the mitral valve at the crux of the heart farther from the cardiac apex than that of the tricuspid valve, two normally placed left ventricular papillary muscles, mitral semilunar continuity, a typical elliptical, smooth septal wall, and a fishmouth appearance of a mitral valve having two commissures. In the absence of typical offsetting of the AV valves and with cardiac malposition, the trabecular pattern of the ventricles sometimes can be recognized: the smooth wall pattern of the left ventricle and the coarser, more heavily trabeculated pattern of the right ventricle. The appearance of the ventricular outflow tracts may aid in ventricular morphologic diagnosis and should be observed as part of the segmental approach. Normally, there is continuity between the mitral valve of the left ventricle and the aortic valve, but muscle of the right ventricular outflow tract separates the tricuspid and pulmonary valves. The most reliable criterion for identifying the morphologic right ventricle is tricuspid valve chordal attachments to the septum. With an atrial septal defect of the primum type, the AV valves are at the same level (Figure 23-2).
Segmental Analysis: Ventriculoarterial Connection
Ventriculoarterial connection is the manner in which the great arteries and semilunar valves connect to the ventricular outflow tracts. Normally, the morphologic right ventricle connects to the pulmonary valve and the morphologic left ventricle connects to the aortic valve. Four possibilities exist: concordant (normal); discordant (right ventricle to the aorta and left ventricle to the pulmonary trunk); double outlet (usually the right ventricle); and single outlet (aortic or pulmonary atresia or truncus arteriosus).
The most common type of abnormality of ventriculoarterial connection is transposition of the great arteries, in which the morphologic right ventricle gives rise to the aorta and the morphologic left ventricle gives rise to the pulmonary trunk (ventriculoarterial discordance) (Figure 23-3). To diagnose this abnormality, the great vessels must be identified. The pulmonary artery is identified by its branching pattern into left and right pulmonary arteries and ductus arteriosus, and the aorta is identified by the coronary, carotid, and subclavian arteries. Both great vessels may originate from one ventricle (usually the morphologic right ventricle), creating a double-outlet right ventricle. If the aortic or pulmonary valve is atretic, a single-outlet ventricle is the result. Another example of a single outlet is truncus arteriosus, in which a single truncal valve originates from the ventricular mass but overrides the ventricular septum. The ventriculoarterial connection is designated as a single outlet with an overriding truncal valve. In complex malformations, including right atrial isomerism with the asplenia syndrome, the AV septal defect often is associated with a double-outlet right ventricle. In cases of tetralogy of Fallot, overriding of the aortic valve is often present so that almost half of the valve annulus appears to arise from the right ventricle. Mitral aortic continuity is present and, except for the rare circumstance in which more than 50% overriding of the aortic valve occurs, the ventriculoarterial connection in tetralogy of Fallot is concordant.
Ventricular and Atrial Septa
Atrial Septum
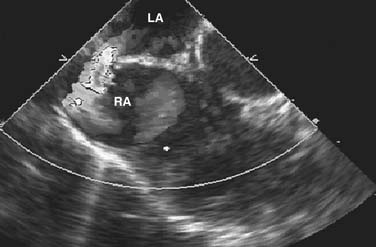
A thin strand of tissue in what appears to be a common atrium suggests right atrial isomerism. The upper atrial septum where a sinus venosus defect may occur can be difficult to evaluate in an older child, but color flow mapping has improved the accuracy of diagnosis in all forms of atrial septal defect (Figure 23-4; also see Figure 23-9).
< div class='tao-gold-member'>

Full access? Get Clinical Tree