Chapter 83 Critical Illness Involving Children Undergoing Hematopoietic Progenitor Cell Transplantation
The first successful human bone marrow transplant occurred in pediatrics for severe combined immunodeficiency. Reported in 1968, the patient received marrow from a human leukocyte antigen (HLA)-matched sibling.1 Presently, in adults and children, the majority of allogeneic transplants are performed for the treatment of malignant disorders such as leukemias and lymphomas, although the field continues to expand to include nonmalignant disorders such as autoimmune disorders, metabolic diseases, immune deficiencies, and hemoglobinopathies. Since its inception, the field of pediatric HPCT has demonstrated vast improvements in morbidity and mortality related to transplantation; however, there are still many hurdles that we need to overcome. The major barriers that still contribute significantly to the morbidity and mortality of allogeneic transplantation are relapse of disease, toxicity from treatment, infection, and graft-versus-host disease (GVHD).
Sources of Hematopoietic Progenitor Cells and Identification of Donors
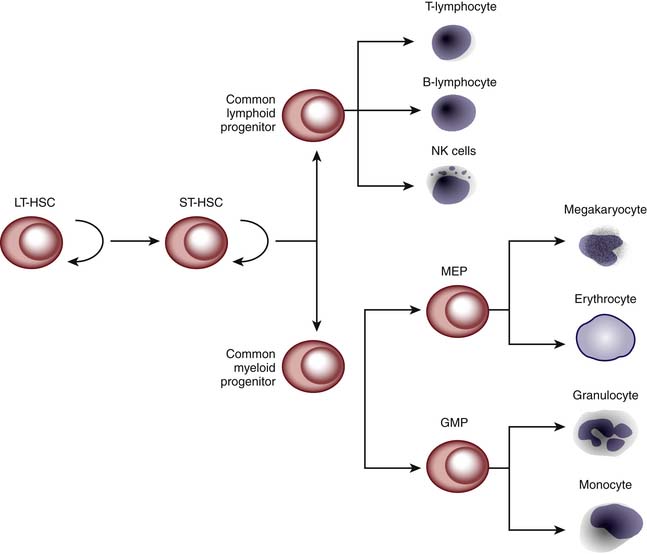
HPCT involves transplanting hematopoietic progenitor cells from a donor source into a recipient. These stem cells are capable of self-renewal and terminal differentiation that ultimately give rise to myeloid cells, lymphocytes, erythrocytes, and platelets (Figure 83-1). The donor source of these stem cells can be from the patient/recipient themselves (autologous) or from another individual (allogeneic). The source of the donor (autologous vs. allogeneic) is generally dependent on the indication for which the transplant is being performed. Autologous transplants can be used for the treatment of nonhematologic malignant diseases, whereas allogeneic transplants are used for hematologic malignancies and marrow failures or dysfunction. Traditionally, HPCT has been performed using stem cells obtained from bone marrow. However, stem cells can be mobilized into the peripheral blood by the use of cytokines (e.g., granulocyte-colony stimulating factor [G-CSF]) or upon recovery from chemotherapy. These peripheral blood stem cells (PBSCs) allow for faster hematopoietic recovery and possibly less tumor contamination than bone marrow for autologous transplantation. However, there may be more side effects in the allogeneic setting (increased incidence of graft versus host disease).2 Umbilical cord blood has also been shown to contain large numbers of stem cells capable of reconstituting hematopoiesis. The first HPCT using cord blood was performed in 1988 on a 5-year-old child with Fanconi anemia using the patient’s HLA-identical sibling.3 Since then, unrelated cord blood stem cells have been used and numerous public cord blood banks have been established worldwide. In circumstances in which there is no matched unrelated donor or cord blood found in a timely fashion, a haploidentical transplantation can be performed using a parent or a sibling. Histoincompatibility barriers of a mismatched transplantation are overcome by using mega-doses of stem cells. However, for this to be successful, a majority of the T cells have to be removed from the graft to prevent severe GVHD. Unfortunately, this increases the risk for severe infection and relapse of the patient’s original disease.4,5
HLAs are expressed on the surface of various cells, in particular white blood cells (WBCs). These antigens are also known as the major histocompatibility complex with relevant genes on the short arm of chromosome 6.6 This genetic region has been divided into chromosomal regions, called classes. Classes I and II are important in transplantation.
Class I is made up of HLA-A, HLA-B, and HLA-C and class II is made up of HLA-DR, HLA-DP, and HLA-DQ, as well as variations on these genes. Traditionally, the loci critical for matching for a bone marrow donor are HLA-A, HLA-B, and HLA-DR. HLA-C and HLA-DQ have recently gained importance and are now considered in determining the best available donor.6,7 Ideally a matched sibling donor is the best donor for a patient. However, only 25% of patients with siblings are fortunate to have a matched sibling donor. If there is no sibling donor, an alternative donor is identified using the National Marrow Donor Program (NMDP) that has approximately 9 million potential donors and nearly 150,000 cord blood units available for patients who need a hematopoietic cell transplant (bone marrow, PBSC, or cord blood transplant).8 As the degree of mismatch between patient and donor increases, so do the risks of complications from transplant, especially GVHD and rejection of the graft.
Indications and Outcomes
HPCT has been used for a variety of diseases. Autologous transplantation has traditionally been used to treat nonhematologic malignant diseases by escalating the doses of chemotherapy to myeloablative doses to hopefully eradicate the cancer. Recently, successive (two or three) autologous transplants have been performed with nonmyeloablative doses of chemotherapy particularly in brain tumors. The rationale of giving hematopoietic stem cells after the chemotherapy is completed is to minimize the period of neutropenia that will hopefully reduce the number of infections.2
Allogeneic HPCT has been performed for hematologic cancers that in children are most commonly leukemias including acute lymphoblastic leukemia and acute myelogenous leukemia. It has also been used to treat hematologic diseases including sickle cell anemia, thalassemias, and severe aplastic anemia. A variety of immune deficiencies and metabolic disorders have been cured by allogeneic transplant including severe combined immune deficiency and hemophagocytic lymphohistiocytosis (Table 83-1).2
Table 83–1 Indications for Pediatric Hematopoietic Progenitor Cell Transplantation
| AUTOLOGOUS TRANSPLANTATION |
| Malignant Disorders |
| Nonmalignant Disorders |
| Autoimmune disorders |
| ALLOGENEIC TRANSPLANTATION |
| Malignant Disorders |
| Nonmalignant Disorders |
Survival from HPCT has improved in the recent years. Typically for autologous transplantation the incidence of treatment related mortality is less than 10%; however, the majority of failures of transplant are due to recurrent disease. Specifically the event-free survival rate for autologous HPCT for high risk neuroblastoma ranges from 33% to 57%.9–11 For recurrent or refractory non-Hodgkin lymphoma the event-free survival in autologous HPCT ranges from 27% to 59%.12 In relapsed or refractory Hodgkin disease the event-free survival ranges from 20% to 62%.12 The NMDP and the Center for International Bone Marrow Transplantation Research data from 1998 to 2006 for acute lymphocytic leukemia shows the probability of survival at 5 years in matched sibling allogeneic HPCT for early or intermediate disease is approximately 50% versus 25% for advanced disease.13 For unrelated donors the 5-year survival for pediatric patients is significantly increased for patients in first complete remission (CR1) (54%) and second complete remission (CR2) (49%) compared with patients with advanced disease (26%) (log-rank P < .0001).14 Advanced disease is defined as third complete remission or refractory disease. For acute myelogenous leukemia the 5-year survival for pediatric patients (<18 years of age) transplanted for acute myeloid leukemia with unrelated donors is significantly increased for those patients transplanted in CR2 compared with those transplanted in CR1 or with advanced disease. However, a greater percent of patients transplanted in CR1 had poor-risk cytogenetics compared to those patients who were transplanted in CR2 (log-rank P < .0004). The 5 yr survival rates are 31% for CR1, 51% for CR2, and 29% for advanced disease.15 However, the data for pediatric patients with AML in CR1 with a matched sibling shows a survival rate at 5 years of 65%.16
For patients with Fanconi anemia and severe aplastic anemia, the 5-year survival is 55% for allotransplants with unrelated donors facilitated by the NMDP in children younger than 18 years.17 Pediatric survival rates after matched sibling HPCT for SAA are now excellent, 85% and even higher in some series.18 In Wiskott-Aldrich disease and severe combined immune deficiency, the 5-year survival is approximately 75% to 80%.19,20 For inherited metabolic disorders, the overall survival of pediatric patients with adrenoleukodystrophy/metachromatic leukodystrophy is approximately 50% and for Hurler syndrome is approximately 60% when using unrelated donor transplants facilitated by the NMDP from 1998 to 2006 (Table 83-2).21
Transplant Procedure
Stem Cell Harvesting/Collection/Cryopreservation
Peripheral blood stem cells can be mobilized in patients recovering from chemotherapy (autologous) or by giving allogeneic donors cytokines such as G-CSF. Their stem cells then can be collected using an apheresis machine in an outpatient setting. Collection of sufficient cells for transplantation may require several apheresis procedures. Stem cells for allogeneic transplantation usually are collected on the day they are anticipated to be reinfused into the patient. Autologous collection of stem cells requires cryopreservation of the cells until the day of reinfusion. Dimethyl sulfoxide is added to the collection product to ensure cell viability, and the cells are frozen in liquid nitrogen until needed.
Complications
Patients undergoing HPCT are at high risk for complications that may require a stay in the pediatric intensive care unit (PICU). In one series, 19% of pediatric HPCT patients required a PICU admission.22 In other published series, 6% to 25% of pediatric HPCT patients required mechanical ventilation.23,24 Because of the high use of critical care services by HPCT patients, it is beneficial for the pediatric intensivist to be familiar with their complications.
The reasons for these patients being at such high risk for critical illness are multifactorial. Many of these patients are undergoing HPCT for an underlying disease that places them at risk for critical illness such as malignancies, severe immunodeficiencies, and metabolic disorders. To make room for the new hematopoietic progenitor cells, patients are given conditioning regimens with high doses of toxic chemotherapy and/or radiation. This makes them severely immunocompromised; placing them at high risk for opportunistic infections. The conditioning agents themselves cause significant oxidative stress, and may be the common denominator behind many of these complications.25
While mortality rates for HPCT patients requiring ICU care are quite high in comparison to the general ICU population, they appear to be improving. Data from the 1980s showed mortality rates for mechanically ventilated pediatric HPCT patients to be near 90%.24,26 However, more recent data indicate the mortality rates to be closer to 60%.24,27,28 In a single institution report, HPCT patients requiring vasopressor or inotropic support due to sepsis had PICU mortality rates of 30%. In the subgroup of septic patients requiring both inotropic and/or vasopressor support and mechanical ventilation, mortality rates were 74%.29 Some of the improvements seen in outcomes over the years may be due to differing characteristics of the patients, as very few studies reported severity of illness scores.26 In any case, no series reporting on mortality of pediatric HPCT patients was able to predict with 100% certainty that a given patient would not survive. Therefore it remains up to the critical care team in conjunction with the transplant team to use their best judgment when making recommendations to families regarding appropriateness and duration of critical care services for this complex patient population.
Cardiac Complications
Cardiac complications following HPCT can occur acutely during the immediate transplant period or as a late sequelae in survivors. The heart may be injured during the transplant process from a variety of pathophysiological etiologies.30,31 First, previous cardiotoxic treatments and therapies such as anthracyclines and iron overload from frequent red cell transfusions may predispose the heart to subsequent injury during transplantation. In addition, cardiotoxic therapies such as cyclophosphamide and irradiation used as part of the preparative regimen may further injure the recipient heart.32,33 Moreover, hyperhydration therapies, blood product transfusions, and impaired renal function may place further stress on the heart. Finally, sepsis, that commonly affects the HPCT patient, has been found to decrease cardiac contractility.34
Despite these potential cardiac problems, cardiac complications are relatively rare in the early posttransplant period after HPCT. In an analysis of 2821 adult and pediatric patients, only 26 (0.9%) experienced a major or fatal cardiac complication in the first 100 days after transplant.35 Seven of the 26 cardiac complications occurred in children, and given the median age of 22 years in that trial, an incidence of approximately 0.5% may be inferred for pediatric HPCT recipients. Among the 26 patients with significant cardiac complications, 11 had evidence of heart failure, 5 had pericardial tamponade, and 10 had dysrhythmias.35 All 11 patients with heart failure died compared with only one each with tamponade or a dysrhythmia. All cases of heart failure occurred between day −6 and day +35. Four of the seven pediatric patients had heart failure. In another report, the Associazione Italiana Ematologia Oncologia Pediatrica-BMT Group described their transplant-related toxicity in 636 pediatric patients transplanted for acute leukemia.36 In their experience, the incidence of moderate or severe cardiac toxicity in the first 90 days posttransplant varied by the type of transplant with autologous recipients experiencing an incidence of 1.9% (4 in 216, two deaths) and allogenic recipients of a compatible related donor experiencing a comparable incidence of 2.4% (7 in 294, four deaths). However, recipients of an allogeneic alternative donor experienced a 6.4% rate of these cardiac complications (8 in 126) with all eight experiencing an early death. In that study, the presence of moderate or severe cardiac toxicity increased the relative risk of an early posttransplant death more than ninefold (relative risk, 9.1; 95% confidence interval, 2.8 to 29.6), and more so than any other organ system toxicity.
The manifestations of the cardiac disease are varied and include myocardial ischemia, dysrhythmias, pericardial effusion, pericarditis, and progressive congestive heart failure. An accepted scoring system based on previously published grading of cardiac toxicities following stem cell transplantation consists of the following.35
Late cardiovascular toxicity occurring a year or more after HPCT is also being reported.37,38 Late cardiovascular complications following HPCT include heart failure, dysrhythmias, hypertension, and cerebrovascular accidents. Several pathologic mechanisms of late congestive heart failure have been offered including those of acute heart failure such as previous cardiotoxic agents (anthracyclines, alkylating agents, thoracic irradiation) in conjunction with cyclophosphamide and total body irradiation during conditioning regimens. However, other factors such as the presence of chronic GVHD and patient characteristics such as age, size, and gender may play a role in late congestive heart failure. In one report of 155 long-term pediatric survivors of HPCT with a median length of follow-up of 9 years, 14 were found to have hypertension, 4 were found to have abnormal asymptomatic electrocardiograms, and 4 were found to have abnormal asymptomatic echocardiograms.38 All patients in that report had a left ventricular shortening fraction greater than 30%. In another report of 112 children who received an allogeneic HPCT and survived at least 1 year, 11 had abnormal echocardiographic findings, 8 had hypertension, and 2 had cerebrovascular accidents.39 The probability of developing a cardiovascular complication at 10 years was 11% ± 3% in that report. Moreover, among patients who developed a cardiac complication, all had received total body irradiation. Total body irradiation both alone, and in conjunction with pretransplant anthracycline use, was also associated with a negative impact on cardiac function 5 years after transplant in a cohort of 162 pediatric patients who underwent allogeneic HPCT for both malignant and nonmalignant diseases.40 In that study, 14 (12%) of the 119 patients with pretransplant echocardiograms were found to have abnormal shortening fractions at the time of transplant. The cumulative incidence of shortening fraction abnormalities increased to 26% by the fifth year of follow-up in that report. In another study, assessing cardiac function during cardiopulmonary exercise testing revealed a significantly decreased maximal cardiac index in 33 children who had undergone HPCT in longitudinal follow-up.41 Although no patient experienced a dysrhythmia or had electrocardiographic evidence of ischemia, four patients were found to have a shortening fraction less than 28%. Based on the finding of a decreased maximal cardiac index and a normal peak heart rate, the authors concluded that this provided evidence of long-standing subclinical cardiac dysfunction in this patient population.
Pulmonary Complications
In adults the incidence of pulmonary complications after HPCT ranges from 30% to 60%.42 The incidence in children is reported between 12% and 25%.43,44 The need for mechanical ventilatory support is the most frequent reason for admission of HPCT patients to the PICU.22,28
Pulmonary complications can be divided into early and late complications (Table 83-3). Early complications occur within the first 100 days after transplant. The division into early and late complications is not absolute but may help the clinician in developing a differential diagnosis. Early complications include infection, diffuse alveolar hemorrhage (DAH), engraftment syndrome causing pulmonary edema, and idiopathic pneumonia syndrome.45 Late-onset complications occur beyond 3 months after HPCT and include bronchiolitis obliterans (BO), bronchiolitis obliterans organizing pneumonia (BOOP), and idiopathic pneumonia syndrome (IPS).46
Table 83–3 Pulmonary Complications of Hematopoietic Progenitor Cell Transplantation
| Complications | Characteristics | Treatment |
|---|---|---|
| EARLY-ONSET PULMONARY COMPLICATIONS | ||
| Infection | Positive test for infection | Antimicrobials |
| Diffuse alveolar hemorrhage | Progressive bloody return on BAL | Corticosteroids, FFP, plasmapheresis |
| Idiopathic pneumonia syndrome | Diffuse noninfectious lung injury | Etanercept |
| Engraftment syndrome | Periengraftment pulmonary edema | Corticosteroids |
| LATE ONSET PULMONARY COMPLICATIONS | ||
| Bronchiolitis obliterans | Obstructive lung disease | Corticosteroids, macrolides |
| Bronchiolitis obliterans organizing pneumonitis | Restrictive lung disease | Corticosteroids |
| Idiopathic pneumonia syndrome | Diffuse noninfectious lung injury | Etanercept |
| Pulmonary veno-occlusive disease | Pulmonary hypertension | Sildenafil, prostacyclin, defibrotide |
BAL, Bronchoalveolar lavage; FFP, fresh frozen plasma.
Early Pulmonary Complications
Engraftment Syndrome
Engraftment syndrome occurs just as patients begin to show signs of neutrophil recovery. This syndrome is likely caused by pulmonary leukoagglutination and inflammatory cytokines. Patients may develop fever, rash, fluid retention, capillary leak, and pulmonary edema. In severe cases patients can develop multiorgan involvement.47 Engraftment syndrome may be related to a graft-versus-host response or in some cases a host-versus-graft response. In mild cases, no treatment is necessary.47 In more severe cases, particularly if there is lung involvement, corticosteroids can be very beneficial.47–49
Diffuse Alveolar Hemorrhage
Alveolar hemorrhage may be infectious or noninfectious in etiology. However, the term DAH in an HPCT patient generally refers to a noninfectious etiology. The reported incidence ranges from 1% from 21% of HPCT patients. It usually occurs in the early posttransplant period and is characterized by widespread alveolar injury, absence of infection, and progressively bloodier return of BAL fluid during bronchoscopy.50 The exact etiology of DAH is unknown but like other noninfectious pulmonary complications it is associated with GVHD and engraftment.51–53 Endothelial injury from chemotherapy and radiation, inflammation, undiagnosed infections and immune mediated damage related to GVHD have all been postulated as the cause.50,52
Successful use of high dose corticosteroids has been reported in case reports of DAH.50,54 However no prospective studies have proven the benefit of this therapy.50,52 Fresh frozen plasma transfusions and plasmapheresis have been tried but are of uncertain benefit.50 Aminocaproic acid used in conjunction with corticosteroids has been described in a series of eight patients with DAH refractory to corticosteroids. The overall 100-day mortality for these eight patients was 44%, an improvement over those treated with steroids alone where the overall 100-day mortality was 83%.55
Idiopathic Pneumonia Syndrome
Incidence and Diagnosis
The incidence of IPS in pediatric HPCT patients has been reported between 2% and 11.8%.44,46,56 IPS is usually considered an early complication of transplant, but has also been described as a late complication of transplant. The diagnosis is made by meeting the diagnostic criteria set by an expert panel convened by the NIH in 1993. Patients must show evidence of widespread lung injury as evidenced radiographically by bilateral lung disease, signs and symptoms of pneumonia (cough, dyspnea or rales), abnormal lung function (increased alveolar to arterial oxygen gradient, pulmonary function testing with restrictive lung disease), and absence of an infectious etiology.57
The term IPS is often used interchangeably with idiopathic pneumonitis and interstitial pneumonitis in the literature. However, interstitial pneumonitis is the histopathologic description in some cases of IPS. Other cases of IPS will show histopathologic findings consistent with DAH or BOOP.57 In any case, IPS, interstitial pneumonitis, DAH, BOOP, and BO are all considered noninfectious pulmonary complications of transplant. As prevention and treatment of infectious pulmonary complications have improved, these noninfectious complications are now the more troublesome.42
The literature continues to be confusing regarding the nomenclature of these noninfectious pulmonary complications of transplant. It is important for the critical care physician at the bedside to treat their patient appropriately without becoming anguished over the particular name of the disease. Though steroids and etanercept (tumor necrosis factor-alpha [TNF-α] receptor antagonist) may prove to be beneficial, at the present time no large clinical trials have shown any therapy to be effective for any of these noninfectious pulmonary complications. Therefore, paying close attention to fluid balance and utilizing lung protective strategies during mechanical ventilation are likely the most important aspects of care we can provide.
Etiology of Idiopathic Pneumonia Syndrome
Inflammation likely plays a significant role in the development of IPS. GVHD is known to be associated with high levels of inflammatory cytokines. GVHD has consistently been shown to be associated with IPS.46,56,59 Because of this association, there is debate in the literature if IPS actually represents GVHD of the lung.57
In the mouse models of IPS when TNF-α knockout mice are used as recipients, the mice develop IPS like wild type mice. However, when TNF-α knockout mice are used as donors for wild type recipients, the severity of IPS is significantly less. In this model, TNF-α is derived from donor T-cells.60 This animal model fits the clinical observation that patients who receive T-cell-depleted grafts have a lower incidence of pulmonary complications.58,61
Some cases of IPS may be caused by an unidentified infection. A retrospective study of stored BAL samples from HPCT patients with pulmonary complications detected human metapneumovirus in 5 (3%) of 163 patients. Three of the five patients were diagnosed with IPS. On review of these patient’s medical records, they initially presented in the first 40 days after transplant with typical upper respiratory symptoms: fever, cough, nasal congestion, and sore throat. Four of the five patients died of rapidly progressive acute respiratory failure. Three of the five had DAH.62
Treatment of Idiopathic Pneumonia Syndrome
Because of the role of inflammation in IPS, corticosteroids have been used as therapy but have not been shown to be efficacious.46,63 Therefore, since TNF-α has been shown to be an important mediator in mouse models of IPS, the soluble TNF-α-binding protein, etanercept has been used in recent years. It is currently under investigation in a large multicenter trial of both pediatric and adult patients who develop IPS from HPCT. Preliminary results look promising with 10 of the first 15 patients reported to have a complete response. The 28-day survival rate is 73% thus far.64 This is a significant improvement from previous reported mortality rates approximating 50%.46,58,64
Continuous veno-venous hemofiltration has also been used in patients with acute lung injury after chemotherapy and HPCT. In a series of 10 patients, seven had a noninfectious lung injury. Four of these 7 were HPCT patients. All patients were mechanically ventilated and met criteria for ARDS. Three of the four HPCT patients with noninfectious lung injury survived to extubation.65
Lung transplantation has been reported in four children who have developed chronic respiratory failure as a complication of HPCT. Of the four patients reported, two are alive without significant complications 2 and 7 years after lung transplant.66
Calfactant (calf lung-derived surfactant) may play a role in the treatment of acute lung injury after HPCT. A post hoc analysis of a multicenter trial of calfactant in mechanically ventilated pediatric patients with acute lung injury showed possible benefit in the subgroup of immunocompromised patients. Twenty-seven of the 52 immunocompromised patients analyzed had undergone HPCT. In this subgroup analysis, patients who received calfactant had a 50% mortality rate, whereas those receiving placebo had a 60% mortality rate.67 A trial of calfactant in pediatric patients with acute lung injury with leukemia, lymphoma, or history of HPCT is currently underway.
Extracorporeal membrane oxygenation (ECMO) has infrequently been used as a heroic measure for HPCT patients with severe lung injury. In a recently published review of the Extracorporeal Life Support Organization database there were no ECMO survivors with a history of recent HPCT.68 However, there is one case report in the literature of an 8-month-old girl with SCIDS who was transplanted while symptomatic with bronchiolitis. ECMO was used successfully as a bridge to engraftment.69
Late Pulmonary Complications
Bronchiolitis Obliterans/Bronchiolitis Obliterans Organizing Pneumonia
BO and BOOP are late onset noninfectious pulmonary complications of HPCT. Both of these complications are associated with chronic graft-versus-host disease.61,70 Patients with BO have an obstructive pattern on pulmonary function testing. On high resolution chest computed tomography (CT) both high and low attenuation areas are seen as are bronchial dilatation, bronchial thickening, vascular attenuation, and expiratory air trapping. Biopsy specimens show submucosal bronchiolar fibrosis and luminal narrowing and obliteration.70
Patients with BOOP have patchy air space disease on chest radiograph. High-resolution chest CT shows ground-glass opacifications, areas of consolidation, and pulmonary nodules.70 As opposed to patients with BO, pulmonary function testing in BOOP shows a restrictive lung disease pattern. Biopsy specimens of BOOP show granulation tissue in the distal airways, alveolar ducts and peribronchial alveolar space.42,71
For both BO and BOOP it is recommended that patients undergo bronchial alveolar lavage to rule out infection. BO may be diagnosed on clinical grounds to avoid open lung biopsy and its associated risks. The diagnosis of BOOP generally requires biopsy. However, unlike BO, a transbronchial specimen is often sufficient.70
The number of patients with BO or BOOP reported in the literature is small. Therefore, it is difficult to make firm recommendations regarding treatment or prognosis. However, these complications may respond better to steroids than other noninfectious pulmonary complications of HPCT.42,46 Macrolide therapy may be of benefit in patients with BO after HPCT but requires further study.72 Other therapies such as inhaled cyclosporine, etanercept, infliximab and extracorporeal photochemotherapy have been described in case reports for BO.70
Pulmonary Veno-occlusive Disease
Case reports of pulmonary veno-occlusive disease (PVOD) in HPCT patients have been infrequently described. Patients have presented both early and late after HPCT. Patients present with increasing dyspnea and signs of right heart failure. Cardiomegaly and pulmonary edema are seen on chest x-ray. Pulmonary hypertension is seen on echocardiogram. In patients who have undergone cardiac catheterization, high right atrial pressure, right ventricular pressure, and pulmonary artery pressures are seen, whereas the pulmonary artery wedge pressure is frequently normal. Pathologic specimens show fibrosis of the venules and small pulmonary veins, whereas the larger pulmonary veins are typically normal. Because the resistance to flow in the pulmonary veins is typically normal in PVOD, the pulmonary artery wedge pressure appears normal despite having increased resistance through pulmonary venules and small pulmonary veins. Pulmonary arterial intimal fibrosis and hypertrophy may also be seen. Steroids, other immunosuppressive agents, and anticoagulation have been used without notable benefit. Sildenafil with prostacyclin has been reported to be of some benefit in treating the pulmonary hypertension. Defibrotide may be beneficial given its efficacy in hepatic VOD, but there are no available reports of its use in patients with pulmonary VOD.73,74
Dilemmas in the Diagnosis of Pulmonary Complications
St. Jude Children’s Hospital recently published data regarding the diagnostic yield of BAL at their institution.75 BAL identified the cause of respiratory symptoms in 53 (67.9%) of 78 of their allogeneic patients and 7 (63%) of 11 autologous patients. The most common finding diagnosed on BAL was bacterial infection (52%). The patients tolerated the procedure well with complications noted in less than 20%. In their series, transbronchial biopsy added additional information in only two of seven patients. They also noted that 14 of 16 patients who underwent open lung biopsy already had a positive BAL. The authors concluded that BAL had a beneficial risk/benefit profile and was useful in identifying patients who had an infectious etiology to their lung injury. However, biopsy did not add significantly more information but carried an unacceptable morbidity rate of 47%.
In contrast, a second study from Taiwan looked at the diagnostic yield of open lung biopsy versus BAL in a cohort of both pediatric and adult BMT patients with diffuse pulmonary infiltrates.76 They found open lung biopsy resulted in a change in clinical management in 63% of their patients while BAL only offered a diagnosis in 30.7% of patients. This series found a shorter duration of intubation in patients who underwent open lung biopsy as opposed to BAL. The authors concluded this was due to a more accurate diagnosis leading to appropriate treatment in the open lung biopsy patients. In this cohort of patients the most common diagnoses were idiopathic interstitial pneumonitis and cytomegalovirus (CMV) pneumonitis as opposed to the St. Jude cohort where bacterial pneumonia was the most common. The difference in underlying diagnoses could explain the differences seen in the utility of BAL versus open lung biopsy.

Full access? Get Clinical Tree