CHAPTER 8
Coronary Artery Disease
Joseph J. DeRose, Sr., MD • Joseph J. DeRose, Jr., MD
Epidemiological studies have demonstrated a substantial decline in cardiovascular mortality in industrialized countries over the past two decades (World Health Organization [WHO], 2011). This decline can be attributed to improvements in primary prevention and the management of acute coronary syndromes (ACS; Steg & Dorman, 2011; WHO, 2011). Despite this spectacular progress, cardiovascular disease remains the number one cause of mortality worldwide and in low- and middle-income countries. Epidemic obesity and metabolic syndromes in North America appear to be challenging this trend toward reduced mortality. However, the age- and sex-adjusted rates of acute myocardial infarction (MI) continue to diminish steadily in the United States (Yehrw, Chandra, Sorel, Selbr, & Co, 2010).
Knowledge of the etiology, pathogenesis, clinical manifestations, diagnosis, and treatment of coronary artery disease (CAD) has grown considerably. It remains the responsibility of the primary care provider not only to recognize patients with cardiac chest pain but also to identify and treat risk factors associated with CAD in asymptomatic patients. It is hoped that preventive guidelines will reduce both the morbidity and mortality rates of this disease.
 ANATOMY, PHYSIOLOGY, AND PATHOLOGY
ANATOMY, PHYSIOLOGY, AND PATHOLOGY
Coronary Anatomy
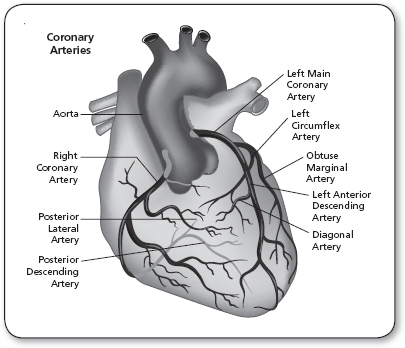
The right coronary artery (RCA) and left main coronary artery are the two major arterial trunks that originate from the aortic root to supply the myocardium. Functionally, the coronary circulation is divided into the RCA, which perfuses the right ventricle; the left anterior descending (LAD) branch of the left main coronary artery, which supplies the anterior wall of the left ventricle and the anterior septum; and the left circumflex branch (LCx) of the left main coronary artery, which perfuses the lateral wall of the left ventricle (Figure 8.1). These are the three territories that clinicians frequently refer to as “triple-vessel disease” when describing coronary artery pathology.
The coronary circulation is referred to as “right dominant” when the major vessel supplying the posterior aspect of the left ventricle, the posterior descending artery, originates from the RCA. In contrast, a “left dominant” coronary circulation is one in which the LCx gives rise to the posterior descending artery. Seventy-five percent of patients have a right dominant circulation, 15% of patients are left dominant, and 10% of patients have a balanced coronary circulation (Kouchoukos, Blackstone, Hanley, & Kirklin, 2013).
The arteriosclerotic process usually affects multiple coronary arteries. Among all patients undergoing coronary angiography, 40% have all three vessels affected; approximately 30% have disease in two vessels. The main trunk of the left coronary artery has a significant stenosis in 10% to 20% of patients undergoing angiography (Gensini, 1975). The disease process usually affects the proximal portions of larger coronary arteries at or just beyond the sites of branching arteries. When the disease is more extensive, the secondary distal branches of the larger coronary arteries may be affected, rendering them unsuitable for interventional or surgical revascularization procedures (Gensini, 1975; Kouchoukos et al., 2013).
Arterial Physiology
The arterial tree is more than just a series of conduits through which blood travels to the various organs. Rather, both normal and diseased arteries are capable of complex biological processes involved in hemostasis, cytokine and growth factor secretion, permeability, metabolism of vasoactive substances, connective tissue formation, lipid metabolism, and cellular proliferation.
The normal artery is composed of three layers: intima, media, and adventitia. A single cell layer of endothelial cells lines the luminal aspect of the blood vessel and makes up the intima. Endothelial cell integrity is critical for maintaining a permeability barrier between the blood and the extracellular tissues. Endothelial cells are also responsible for providing an intraluminal nonthrombogenic surface. They secrete a wide range of vasoactive substances, including endothelial-derived relaxing factor, prostacyclin, endothelin, angiotensin-converting enzyme (ACE), and platelet-derived growth factor. A special capacity of endothelium particularly important in thermogenesis is its ability to modify lipoproteins. Low-density lipoproteins (LDLs) can be bound by LDL receptors on endothelial cells, internalized and modified. Modified LDLs can then bind to scavenger receptors on the surface of macrophages to form foam cells, an important contributor to the atherosclerotic plaque (Krieger, 1995).
FIGURE 8.1
Coronary arterial anatomy. The three major arterial distributions include the right coronary artery, the left anterior descending artery, and the left circumflex artery.
The muscular layer of the artery is termed the media and is composed of smooth muscle cells. These smooth muscle cells normally exist in a quiescent state and exhibit a contractile phenotype that is capable of altering blood vessel tone. Like endothelial cells, these smooth muscle cells exhibit LDL receptors and are capable of lipoprotein modification and presentation to macrophages. With overlying endothelial denudation or injury, medial smooth muscle cells are capable of responding to a variety of mitogens and chemoattractants. The quiescent contractile smooth muscle cell can then be stimulated to differentiate into a proliferative phenotype that can migrate into the intima, divide, and secrete extracellular matrix (Krieger, 1995; Ross, 1993; Zhao, 2013).
The outermost layer of the vessel wall, the adventitia, is composed of a thin layer of collagen and fibroblasts. Although once thought to be a biologically inactive layer, it is now recognized that adventitial fibroblasts participate in the processes of atherogenesis and arterial restenosis. Differentiation of adventitial fibroblasts into myofibroblasts can result in remodeling of the vessel wall. Adventitial myofibroblasts may serve to decrease vessel size in the process of atherogenesis and arterial restenosis, not unlike their role in wound contraction during wound healing (Gibbons & Dzau, 1994).
Process of Atherogenesis
Endothelial injury is the inciting event in the generation of luminal stenosis. Disruption of the endothelial lining exposes the circulating blood elements to the underlying thrombogenic surface of the media. Platelets readily adhere to this surface and release mitogens and growth factors. Medial smooth muscle cells are stimulated to migrate into the intima, proliferate, and secrete extracellular matrix, resulting in intraluminal stenosis. Lymphocytes are also attracted to the site of injury, resulting in continued cytokine release and antigen presentation. This response to the injury process is responsible for restenosis after angioplasty and coronary artery bypass grafting (CABG), as well as the transplant atherosclerosis seen in immunologically injured endothelium of heart transplants (Badimon, Fuster, Chesebro, & Badimon, 1993; Gibbons & Dzau, 1994; Ross, 1993).
The cause of endothelial injury in primary atherosclerosis is most commonly secondary to hyperlipidemia (Gibbons & Dzau, 1994; Ross, 1993). Circulating lipoproteins, especially LDLs, can be taken up by endothelial cells and medial smooth muscle cells. After modification and presentation, resident macrophages scavenge the modified LDLs and oxidize them on the luminal aspect of the artery. This fatty streak can be seen as early as late childhood and young adulthood and is anatomically distributed in areas ultimately affected with progressive atherosclerosis.
Oxidation of LDLs by macrophages leads to toxic injury to the endothelium through generation of superoxide radicals. This endothelial injury, together with secretion of numerous growth factors by these activated macrophages, sets into motion the cascade of smooth muscle cell activation. The resulting lesion is a fibrous plaque composed of large numbers of intimal smooth muscle cells, collagen fibers, macrophages, and lymphocytes. Continued lipid uptake results in intracellular and extracellular accumulations of cholesterol esters. Progressive growth of the fibrous plaque results in slow stenosis of the arterial lumen and eventual occlusion.
Endothelial injury in atherogenesis may also occur in response to flow dynamics associated with hypertension, glycosylation associated with diabetes, superoxide production involved in cigarette smoking, and primary viral injury from cytomegalovirus infection (Badimon et al., 1993). Multidetector computed tomography (CT) has emerged as a means of measuring luminal stenosis, coronary calcium, and even the extent of noncalcified coronary plaque volume. The future holds the possibility of molecular imaging based on the knowledge of molecular mechanisms involved in the development and progression of atherosclerotic plaques and a contrast agent that is able to identify different molecules and/or cells in the target zone (Badimon, Ibanez, & Cimmino, 2009).
Thrombosis and Plaque Stability
Continued injury to the endothelium results in worsening endothelial dysfunction. The permeable barrier created by the endothelium is lost, and the balance between intraluminal anticoagulant and procoagulant properties is disrupted (Loscalzo, 1992). Rupture of the endothelial lining of an atherosclerotic plaque can result in intramural hemorrhage, exposure of subintimal collagen, and intraluminal thrombosis. In contrast to the slow luminal reduction caused by the progressing atherosclerotic plaque, plaque rupture and thrombosis result in acute vessel closure. Without the time necessary for compensatory collateral formation and angiogenesis, plaque rupture results in acute myocardial ischemia and potential myocardial cell death. Tissue factor content is increased in unstable angina and correlates with areas of macrophages and smooth muscle cells, suggesting a cell-mediated thrombogenicity in patients with ACS (Moreno et al., 1996).
Coronary Blood Flow and Myocardial Ischemia
Ischemia refers to the inadequate delivery of oxygen to the myocardium, accompanied by an inadequate removal of metabolites consequent to reduced perfusion. Myocardial ischemia is the result of an imbalance between myocardial oxygen demand and myocardial oxygen supply. The therapies for CAD are aimed at reestablishing this balance by decreasing myocardial oxygen consumption, increasing coronary blood flow, or both.
DETERMINANTS OF MYOCARDIAL OXYGEN CONSUMPTION
Cardiac energy generation is primarily aerobic, and therefore, myocardial oxygen consumption is an accurate measure of total cardiac metabolism. Increases in cardiac oxygen consumption are primarily affected by changes in systolic wall tension, contractility, and heart rate (Ardehali & Ports, 1990; Graham, Covell, Sonnenblick, Ross, & Braunwald, 1968; Rooke & Fiegel, 1984).
Systolic wall tension is determined by both stroke volume and systolic pressure generation. These determinants of wall tension have their clinical correlates in preload and afterload. Increases in either parameter result in a greater overall external workload and an increase in myocardial wall tension (Rooke & Fiegel, 1984). By the Laplace relation, myocardial wall tension decreases with decreasing ventricular size and increases with ventricular dilatation.
Changes in contractility increase both systolic pressure generation and time to peak pressure generation; without significant changes in ventricular volume, these translate into increased myocardial wall tension and increased myocardial oxygen consumption (Ardehali & Ports, 1990; Graham et al., 1968; Rooke & Fiegel, 1984). Positive inotropic agents also enhance excitation–contraction coupling. Increased oxygen requirements then result from greater and more rapid calcium uptake by the sarcoplasmic reticulum. Finally, acceleration of the heart rate increases myocardial oxygen demand by increasing the frequency of tension development per unit of time and simultaneously by increasing contractility (Ardehali & Ports, 1990; Rooke & Fiegel, 1984). Myocardial ischemia is the result when any of these determinants causes increased myocardial metabolic demands without concomitant regulation of oxygen delivery.
DETERMINANTS OF MYOCARDIAL OXYGEN DELIVERY
Coronary blood flow is the major determinant of myocardial oxygen delivery. Perfusion of the coronary arteries occurs primarily during diastole because of myocardial compression of the intramyocardial and subendocardial arterioles during systole. Approximately 80% of coronary flow to the left ventricle and 50% of flow to the right ventricle occur during diastole. Coronary perfusion pressure, therefore, is determined by both mean aortic pressure and left ventricular end-diastolic pressure.
Autoregulation of the coronary circulation via neurohumoral mechanisms serves to maintain coronary flow fairly constant over a wide range of perfusion pressures. In the presence of fixed coronary stenosis, however, autoregulation cannot further increase regional blood flow to accommodate increases in myocardial oxygen demand. Coronary vessels with atherosclerotic lesions are maximally dilated to augment distal flow in the setting of luminal obstruction. With further metabolic demands, autoregulation is not possible and regional myocardial ischemia with resultant myocardial dysfunction occurs. Furthermore, in the setting of atherosclerosis, endothelial dysfunction results in impaired ability to generate vasoactive substances such as endothelial-derived relaxing factor and prostacyclin. This results in further impairment of smooth muscle relaxation as well as a loss of platelet aggregation inhibition.
 EPIDEMIOLOGY
EPIDEMIOLOGY
An estimated 15.4 million Americans have CAD (Go et al., 2014). Among adults 30 to 74 years of age, the average 10-year risk for developing CAD is 6.5%. A person’s lifetime risk varies as a result of his or her risk factor profile. For someone with no CAD risk factors, the lifetime risk for developing CAD is 3.6% for men and <1% for women; but with two or more CAD risk factors (e.g., hypertension, hyperlipidemia, diabetes, or smoking), that risk increases to 37.5% for men and 18.3% for women (Go et al., 2014).
Risk Factors
Risk factor assessment and modification are an integral part of the evaluation and treatment of patients with both known and suspected CAD. Unfortunately, risk factors are often misinterpreted as either necessary or sufficient causes of disease. The primary care provider must be mindful of the fact that risk factors represent associations that may or may not be causal. Risk factors can be divided into modifiable and nonmodifiable categories (Table 8.1). This characterization has clinical implications: Only modifiable risk factors can be targeted for preventive measures. However, patients with strong nonmodifiable risk factors may warrant greater intensity of risk factor management because of an increased risk of CAD.
Risk Factors Associated With the Epidemiology of CAD |
NONMODIFIABLE RISK FACTORS FOR CAD |
Male sex Age >45 y Family history Race Socioeconomic factors |
MODIFIABLE RISK FACTORS FOR CAD |
Dyslipidemia Diabetes Hypertension Renal disease Postmenopausal status Cigarette smoking/tobacco use Obesity Physical activity Alcohol intake Psychosocial factors: stress, type A personality, depression Serum homocysteine |
CAD, coronary artery disease.
NONMODIFIABLE RISK FACTORS
Age, Gender, Race, and Family History • Men have a higher risk for CAD than women and men tend to have heart attacks at a younger age than women. After 40 years of age, the lifetime risk of developing CAD is 49% for men and 32% for women. Approximately 80% of the people dying from CAD are 65 years of age or older (Go et al., 2014). People of African American descent have a higher risk of developing CAD. This is likely due to higher prevalence of some risk factors like hypertension and diabetes in this population.
A family history of heart disease is associated with a higher risk of CAD, especially if a first-degree relative developed heart disease at an early age. The risk is highest if a father or a brother was diagnosed with heart disease before age 55 years, or a mother or a sister developed it before age 65 years (World Heart Federation, n.d.). The presence of this very strong nonmodifiable risk factor should prompt the primary care provider to treat aggressively any concomitant risk factors in such a patient.
Socioeconomic Factors • People with lower socioeconomic status or lower level of education are much more likely to develop heart disease than those who are wealthier or better educated. This risk persists even with ongoing progress in addressing traditional risk factors such as smoking, high blood pressure (BP), and elevated cholesterol (Franks, Winters, Tancredi, & Fiscella, 2011).
Neither risk factor modification nor decreased MI rates have been uniform across all socioeconomic groups. Some believe that the higher the level of education and socioeconomic status, the greater is the potential for lifestyle modification. The mechanism of increased risk among people from lower socioeconomic levels may be indirectly related to decreased access to preventative health care and poor participation in a strict risk factor modification program (Govil, Weidner, Merritt-Worden, & Ornish, 2009). Increasing access to lifestyle modification programs should be a goal for primary care providers caring for patients of lower socioeconomic status.
MODIFIABLE RISK FACTORS
Lipids • LDL cholesterol is a major risk factor for heart disease. LDL cholesterol builds up on the inside of artery walls leading to artery blockage and MI. The higher the LDL cholesterol levels, the higher the risk. LDL levels are the main focus of cholesterol-lowering treatment. Traditionally, LDL targets were determined based on a patient’s risk factors, with most patients being recommended to aim for an LDL below 130 mg/dL. In patients with other risk factors for heart disease, the target LDL was below 100 mg/dL. In patients at very high risk of heart disease, recommendations were made for an LDL level below 70 mg/dL. High risk for heart disease includes a previous MI or stroke, carotid artery atherosclerosis, peripheral vascular disease, or diabetes mellitus.
Revised guidelines from the American College of Cardiology (ACC) and the American Heart Association (AHA; Stone et al., 2013; http://circ.ahajournals.org/content/early/2013/11/11/01.cir.0000437738.63853.7a) provide updated guidance to providers on how to best manage the care of individuals at risk of cardiovascular disease. Treatment goals have shifted focus on the intensity of statin therapy based on CAD risk factors.
High-density lipoprotein (HDL) cholesterol is known as good cholesterol. The notion that raising HDL cholesterol levels will reduce cardiovascular events is now unsupported based on negative evidence from clinical trials (Jancin, 2013). HDL may not even play an active role in cardiovascular risk. Raising HDL levels had no impact on major cardiovascular events. However, patients born with HDL cholesterol >80 mg/dL may have cardiovascular event protection and those born with HDL cholesterol <30 mg/dL may be at risk. Lowering LDL with 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) to target a LDL/HDL ratio of <3.5 is known to be a protection against CAD (Behrenbeck, 2012; Vogel & Miller, 2009).
The AHA recommends that a triglyceride level of 100 mg/dL or lower be considered optimal. The AHA does not recommend drug treatment to reach this level, but lifestyle changes such as diet, weight loss, and physical activity are encouraged. See Chapter 9, “Dyslipidemias,” for further discussion and treatment recommendations for the management of high cholesterol.
Diabetes • Diabetes mellitus is a major risk factor for CAD that increases the risk of MI (Centers for Disease Control and Prevention [CDC], 2011). Diabetes is defined by the American Diabetes Association (2014) as a hemoglobin A1c (HbA1c) of 6.5 or greater and prediabetes as an HbA1c between 5.7 and 6.4. Adults with diabetes have a cardiovascular death rate that is up to four times greater than that of nondiabetics. In the United States, it has been estimated that as many as 12.5 million diabetic patients have CAD (Albers, Krichavsky, & Balady, 2006).
In the absence of typical warning signs, such as chest pain, patients with diabetes may suffer from reduced heart circulation that goes undiagnosed compared with nondiabetics. Aggressive risk factor modification is essential for patients with diabetes. Screening for CAD in patients with abnormal ECGs or impaired functional status by cardiac stress testing, nuclear imaging, electron-beam CT scan, or cardiac catheterization can lead to early detection (Chopra & Peter, 2012). Patients with diabetes and CAD should receive aggressive treatment with cholesterol-reducing medications (statins), aspirin, beta-blockers, and ACE inhibitors or angiotensin receptor blockers (ARBs) to reduce the risk of cardiovascular complications. See Chapter 15, “Diabetes Mellitus,” for further discussion and treatment recommendations.
Hypertension • Epidemiological data indicate a strong and consistent link between hypertension and CAD. Hypertension can cause physical endothelial damage and functional impairment of endothelial function. The risk of hypertension cannot be taken in isolation. It frequently coexists with other risk factors such as physical inactivity, obesity, alcohol use, hyperlipidemia, diabetes, and smoking. The presence of these CAD risk factors appears to be both causal and additive for hypertension.
In December 2013, the 2014 Evidence Based Guidelines for the Management of High Blood Pressure in Adults: Report from the Panel Members appointed to the Eighth Joint National Committee (JNC-8) was released (James et al., 2014; http://jama.jamanetwork.com/article.aspx?articleid=1791497). This release occurred 10 years after the publication of JNC-7. These guidelines outline nine specific recommendations for initiating and modifying pharmacotherapy for patients with elevated BP based on evidence taken from randomized controlled trials, the gold standard for establishing efficacy and effectiveness:
1. In patients aged ≥60 years, initiate pharmacologic treatment with a systolic blood pressure ≥150 mmHg or diastolic blood pressure ≥90 mmHg and treat to a goal systolic blood pressure of <150 mmHg and goal diastolic blood pressure of <90 mmHg.
2. In patients aged <60 years, initiate pharmacologic treatment at a diastolic blood pressure ≥90 mmHg and treat to a goal of <90 mmHg.
3. In patients aged <60 years, initiate pharmacologic treatment at a systolic blood pressure ≥140 mmHg and treat to a goal of <140 mmHg.
4. In patients aged ≥18 years with chronic kidney disease, initiate pharmacologic treatment at systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg and treat to goal systolic blood pressure of <140 mmHg and goal diastolic blood pressure of <90 mmHg.
5. In patients aged ≥18 years with diabetes, initiate pharmacologic treatment at systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg and treat to a goal systolic blood pressure of <140 mmHg and goal diastolic blood pressure of <90 mmHg.
6. In the general nonblack population, including those with diabetes, initial antihypertensive treatment should include a thiazide-type diuretic, calcium channel blocker (CCB), an ACE inhibitor, or an ARB. (This recommendation is different from the JNC-7 guideline, in which the panel recommended thiazide-type diuretics as initial therapy for most patients.)
7. In the general black population, including those with diabetes, initial antihypertensive treatment should include a thiazide-type diuretic or CCB.
8. In the population aged ≥18 years with chronic kidney disease (regardless of race or diabetes status), antihypertensive treatment should include an ACE inhibitor or ARB to improve kidney outcomes.
9. If goal blood pressure is not reached within a month of treatment, titrate the dose of the initial drug or add a second drug from one of the recommended classes (thiazide-type diuretic, CCB, ACE inhibitor, or ARB). If goal blood pressure cannot be reached with two drugs, add and titrate a third drug from the list provided. Do not use an ACE inhibitor and an ARB together in the same patient. If goal BP cannot be reached using only the drugs in recommendation 6 because of a contraindication or the need to use more than three drugs to reach goal BP, antihypertensive drugs from other classes can be used.
The JNC-8 guidelines point out that for patients older than 60 years being treated for hypertension with a systolic BP <140 mmHg who are doing well on medications, current medications should not be discontinued to get their BP closer to 150 mmHg (Borgmeyer, 2013).
At the time of this writing, the AHA and the ACC had not reviewed these guidelines. See Chapter 11, “Hypertension,” for further discussion and treatment recommendations.
Renal Disease • More than 26 million Americans have chronic kidney disease, and many more remain undiagnosed (Go et al., 2014). The incidence and prevalence of chronic kidney disease continue to increase in the United States. It is unclear if chronic kidney disease is an independent risk factor for CAD. It is known that people with chronic kidney disease are at a higher risk of cardiovascular events; in fact, many will likely die from a cardiovascular event before they develop end stage renal disease (Sarnak et al., 2003). All patients with chronic kidney disease should be considered being at high risk of CAD and receive aggressive risk factor modification. See Chapter 27 on chronic renal diseases for further discussion and treatment recommendations.
Postmenopausal Status • Heart disease is the leading cause of death in women (CDC, 2013a), with one in four female deaths attributed to heart disease (CDC, 2013b). The risk of developing CAD increases after menopause. Prior to menopause, natural estrogen production decreases a woman’s risk of heart disease. Estrogen’s protective effect comes from its influence on regulating cholesterol levels, its vasodilating effects, and its ability to inhibit vascular injury and prevent atherosclerosis (Mendelsohn & Karas, 1999). Estrogen favorably affects the HDL/LDL cholesterol ratio. The nonlipid cardioprotective mechanism of endogenous estrogen is to maintain the function of the endothelium. It helps reduce inflammation, oxidation of lipoproteins, and activation of platelets, thereby reducing clotting and thrombus formation.
As menopause begins between the ages of 45 and 50 years, endogenous estrogen levels fall and the risk of CAD rises. The natural hormone estrogen is no longer available to have a positive effect on the inner layers of the artery wall, reducing blood vessel flexibility.
The benefits of hormone replacement therapy with either estrogen alone or combined estrogen–progestin in postmenopausal women remain uncertain. Historically, studies on estrogen replacement therapy were observational in nature. Based on these extensive observational data, it was believed that estrogen was cardioprotective and estrogen therapy was routinely prescribed for both primary and secondary prevention of CAD (Martin & Rosenson, 2013). However, the observational nature of these studies raised questions regarding unrecognized biases that could have accounted for the observed cardioprotective effects of estrogen.
More recently, a randomized controlled trial examining the effects of estrogen replacement on CAD prevention, called the Heart and Estrogen/Progestin Replacement Study (HERS-I and -II; Grady et al., 2002; Hulley et al., 1998), as well as other small controlled trials and several meta-analyses, did not confirm a protective effect on the heart (Martin & Rosenson, 2013). The Women’s Health Initiative (WHI) conducted a randomized controlled trial that examined the combined effects of estrogen plus progestin on major disease incidence. This trial concluded that the risks, including an increased CAD risk, exceeded the benefits of combined estrogen plus progestin in postmenopausal women (Rossouw et al., 2002). The WHI’s unopposed estrogen trial did not show any effect on CAD incidence, although the risk for stroke and venous thromboembolism were still greater with the use of unopposed estrogen, as was seen in the combined estrogen–progestin trial (Anderson et al., 2004). Follow-up analysis suggests that there is no increased risk for CAD when hormone replacement therapy is used close to the time of menopause; this risk increases for women who are distant from menopause (Rossouw et al., 2007). Experts agree that estrogen is still a reasonable therapy when used for a short term at the lowest effective dose to reduce menopausal symptoms, but it is not recommended for either primary or secondary prevention of CAD. In the past, short-term therapy (with a goal of symptom management) was defined as that lasting <5 years. This definition is somewhat arbitrary, as there is no general consensus about the duration of short-term versus long-term therapy (North American Menopause Society, 2010). Short-term therapy is now generally considered to last two to three years, and not more than five years (Martin & Manson, 2008).
Tobacco • Cigarette smoking is a factor responsible for about one in every five deaths annually in the United States (National Institutes of Health, 2011). Smoking is the main preventable cause of death and illness in the United States. Smoking affects nearly every organ in the body, including the heart, blood vessels, lungs, eyes, mouth, reproductive organs, bones, bladder, and digestive organs. Evidence shows that quitting smoking does not reduce the amount of CAD caused by smoking, but quitting does reduce the risk of heart attack and death to the levels of nonsmokers (Nakanishi et al., 2013).
Inhalation of cigarette smoke produces a transient and reversible prothrombotic increase in fibrinogen levels, increases platelet aggregation, and decreases the ability of endothelial cells to produce or release prostacyclin (Pamukcu, Oflaz, Onur, Cimen, & Nisanci, 2011). Carbon monoxide in cigarette smoke binds to hemoglobin, reducing its oxygen-carrying ability. This can result in a decrease in exercise tolerance. Nicotine is also a potent agonist for the adrenergic nervous system, resulting in increased coronary tone and vasoconstriction. The enhanced vasoconstriction results in an imbalance between oxygen supply and demand and has been associated with vasospastic angina (Benowitz & Gourlay, 1997).
Exposure to secondhand smoke has immediate adverse effects on the cardiovascular system and can cause CAD. Secondhand smoke results in approximately 46,000 premature deaths from heart disease among nonsmokers in the United States each year (CDC, 2013c). Nonsmokers who are exposed to secondhand smoke, either at home or at work, have a 25% to 30% increased risk of developing heart disease. Even brief secondhand smoke exposure can damage the lining of blood vessels and cause blood platelets to become stickier. These changes can result in a MI. People who already have heart disease are at especially high risk of suffering adverse effects from breathing secondhand smoke and should take special precautions to avoid even brief exposure.
Obesity • It is estimated that 68% of adults in the United States are overweight or obese, with 35% of adults falling into the obese category (Go et al., 2014). This prevalence is higher among some ethnic and racial minority groups (e.g., Hispanics and non-Hispanic Blacks) and in people of lower socioeconomic levels. The incidence of obesity has been on the rise since the later half of the 20th century, as major shifts in diet and lifestyle occurred. People have been moving from plant-based diets to diets higher in proteins and fats while at the same time becoming physically inactive. Obesity is a known risk factor for many chronic conditions such as CAD, hypertension, and diabetes (Jensen et al., 2013).
Obesity is measured by the size of the waist, the ratio of the waist to the hips, and the relationship between height and weight. The last measurement is known as the body mass index (BMI). It is not a perfect way of checking the cardiovascular risk but as the BMI increases, so does the risk of heart disease and stroke. The BMI is calculated by dividing the weight in kilograms by the square of the height in meters (kg/m2). A BMI between 25 and 29.9 kg/m2 is considered overweight. Obesity is defined as a BMI >30 kg/m2. A BMI >22 kg/m2 in persons of South Asian origin may be considered overweight. Women with a BMI >21 kg/m2 may be associated with an increased risk for CAD. Underweight is defined as a BMI below 18.5 kg/m2.
Fat, especially intra-abdominal fat, has a significant impact on metabolism. Intra-abdominal fat can affect BP and cholesterol levels. It also interferes with the body’s ability to use insulin effectively, which can result in the development of diabetes, a major risk factor for CAD. The risk of developing type 2 diabetes and hypertension rises as weight increases. Evidence shows that, globally, 58% of diabetes and 21% of ischemic heart diseases can be attributed to a BMI >21 kg/m2 (WHO, 2002).
Physical Activity • The role of physical activity in the prevention of CAD remains controversial. The exact mechanism of protection is multifactorial. Exercise is important in maintaining ideal body weight, muscle mass, and normal BP, as well as optimizing lipid levels. Regular aerobic exercise decreases both systolic and diastolic pressures, improves the myocardial oxygen supply/demand ratio, lowers triglycerides, raises HDL levels, and decreases platelet aggregation.
The greatest risk reduction is achieved in nonactive and moderately active persons. Intense exercise is associated with decreases in total and LDL cholesterol and increases in HDL cholesterol (Fowler, 2012). Patients who exercise regularly have a decreased incidence of sudden death, although sedentary patients who begin an exercise regimen may be at increased risk of acute MI or malignant ventricular arrhythmia. Therefore, a thorough physical examination is recommended for people older than 40 years before initiating a vigorous exercise program. Likewise, younger patients with hypertension, diabetes, and other associated CAD risk factors should undergo a thorough assessment before undertaking an aggressive exercise regimen, including a baseline ECG. A history of angina or an abnormal ECG should prompt further diagnostic testing before initiating an intensive exercise program.
Alcohol • Patients should be advised that if they drink alcohol they should do so in moderation. This means an average of one to two drinks per day for men and one drink per day for woman (a drink is 12 oz. of beer, 4 oz. of wine, 1.5 oz. of 80 proof spirits, or 1 oz. of 100 proof spirits). Heavy alcohol consumption is associated with a number of health risks such as hypertension, hypertriglyceridemia, obesity, stroke, and breast cancer, as well as cardiomyopathy, cardiac arrhythmias, and sudden cardiac death. There is no evidence that drinking wine or any other alcoholic beverages can replace lowering cholesterol, lowering BP, controlling weight, physical activity, and following a healthy diet. The AHA does not recommend drinking alcohol to prevent CAD (American Heart Association, 2014).
Psychosocial Factors • Psychosocial factors may affect a person’s risk of developing CAD and the course of CAD in patients who already have atherosclerosis. People who are said to have a “type A personality” are characterized as highly competitive, ambitious, and in constant struggle with their environment. Although early studies suggested a link between type A personality and CAD risk, a systematic review has found less consistent evidence linking type A personality to CAD risk (Kuper, Marmot, & Hemingway, 2002). Acute stress from life events such as bereavement or natural disasters has been known to trigger CAD events (Rozanski, Blumenthal, & Kaplan, 1999). In patients with atherosclerosis, acute stress causes vasoconstriction, which can induce angina or an MI. Chronic stresses may also play a role in CAD risk. Chronic stressors such as social isolation and lack of social supports, low socioeconomic status, and work stress have been linked to adverse clinical outcomes (Rozanski, Blumenthal, Davidson, Saab, & Kubzansky, 2005).
A comprehensive review of the literature evaluating the relationship between depression and CAD concluded that depression and CAD have a bidirectional relationship; in other words, CAD can cause depression and depression is an independent risk factor for CAD and its complications (Khawaja, Westermeyer, Gajwani, & Feinstein, 2009). Depression may contribute to sudden cardiac death and increase all causes of cardiac mortality. Depression contributes to an unhealthy lifestyle and poor adherence to treatment (Hance, Carney, Freedland, & Skala, 1996; Khawaja et al., 2009). Because of this correlation, primary care providers should screen all patients at risk of CAD for depression and refer patients for treatment when indicated (Fihn et al., 2012).
Serum Homocysteine • Homocysteine has been identified as a risk factor for ischemic events in prospective observational studies (Fihn et al., 2012). However, a meta-analysis of 12 randomized controlled trials concluded that homocysteine-lowering supplements have not been shown to help further reduce cardiovascular events in patients with a prior history of cardiovascular disease (Bazzano, Reynolds, Holder, & He, 2006). This conclusion was subsequently confirmed by a large double-blinded randomized controlled trial of post-MI patients (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine [SEARCH], 2010). Although folic acid plus vitamin B12 effectively reduce homocysteine levels, these supplements do not reduce major vascular events. These results highlight the importance of focusing on guideline-directed medical therapy and lifestyle modifications that have an evidence base to support their use in the prevention of cardiovascular disease, rather than adding folic acid–based vitamin supplements to lower homocysteine (SEARCH, 2010).
Homocysteinurea is a rare autosomal recessive disorder of methomine metabolism. Untreated homocysteinurea may be complicated by CAD. Therefore, vitamin B6, folate, and vitamin B12 may be used to lower homocysteine and may be of benefit in those with this rare disorder (Kazemi, Eshraghian, Omrani, Lankarani, & Hosseini, 2006).
 DIAGNOSTIC CRITERIA
DIAGNOSTIC CRITERIA
The diagnosis of CAD is based on a history of chest pain or anginal equivalent pain together with diagnostic studies that demonstrate either functional or anatomic coronary obstruction. New wall motion abnormalities on echocardiography may also be used as soft criteria for recent myocardial injury. The diagnosis of MI is based on history, ECG changes, and myocardial markers. The two markers utilized to diagnosis MI are creatine kinase MB (CK-MB) and troponin levels.
The CK-MB test is a relatively specific cardiac marker when skeletal muscle damage is not present. The level peaks in 10 to 24 hours. Because it has a short duration, it cannot be used for late diagnosis of acute MI, but it can be used to suggest infarct extension if levels rise again. The CK-MB level is usually back to normal within 2 to 3 days.
The troponin I test is the most sensitive and specific test for myocardial damage. It has increased specificity compared with the CK-MB test; therefore, troponin is a superior marker for myocardial injury. Differential diagnoses of troponin elevation include acute MI, severe pulmonary embolism causing acute right heart overload, heart failure, or myocarditis. Troponins can also be used to calculate infarct size, but the peak must be measured on the third day. After myocyte injury, troponin is released in 2 to 4 hours and persists for up to 7 days.
 HISTORY AND PHYSICAL EXAMINATION
HISTORY AND PHYSICAL EXAMINATION
History
The diagnosis of CAD is based on a careful, skillful clinical history. The patient should be assessed for a history of CAD risk factors, including diabetes mellitus, smoking, hypertension, hyperlipidemia, and family history of CAD. Patients with cerebrovascular or peripheral arterial disease are more likely to have concurrent CAD. Within the current atmosphere of health care cost containment, a concisely focused outlined history will obviate the need for more costly testing.
Typical stable angina pectoris is described as a viselike, constrictive, crushing, or squeezing type of retrosternal chest pain induced by exertion. In some patients, the quality of discomfort is described as mild pressure or substernal burning. In each instance, the symptoms reach their maximal intensity in a few minutes and then dissipate with the cessation of exercise. Typical angina pectoris is relieved within minutes of rest or by using nitroglycerin. The response to nitroglycerin usually occurs within 3 to 5 minutes and can be a very useful diagnostic test. Nonetheless, it has been clearly established that esophageal pain and other syndromes may also respond to nitroglycerin.
Typical angina may be induced by exertional activities such as walking against the wind, climbing stairs or hills, vigorous arm work, and sexual intercourse. The discomfort can likewise be incited by emotional stress (panic, fear, anger, or anxiety), or it may follow a heavy meal. Anginal pain may even occur nocturnally after lying down (angina decubitus) secondary to increased ventricular filling pressure. The duration of discomfort and its cessation are reproducible for each typical anginal syndrome.
Although the area of discomfort is classically retrosternal, radiation is very common. The regions of discomfort may manifest themselves anywhere between the mandible and the epigastrium. Discomfort can radiate to the shoulders, the jaw, the ulnar surface of the left arm, the right arm, or the outer surface of both arms.
Symptoms of myocardial ischemia other than typical anginal discomfort, such as dyspnea with minimal exertion, fatigue, and faintness, are referred to as anginal equivalents. These symptoms are common in the elderly and in patients at high risk of CAD. The symptoms may be caused by elevation of the left ventricular filling pressure and despite a normal ECG should alert the provider to probable severe ischemic heart disease.
In women, while the most common heart attack symptom is some type of pain, pressure, or discomfort in the chest, it is not always severe nor is it the most prominent symptom. It usually is not the crushing chest pain people associate with a heart attack. Women more commonly have heart attack symptoms unrelated to chest pain, such as neck, shoulder, upper back or abdominal discomfort, shortness of breath, nausea or vomiting, sweating, lightheadedness or dizziness, or unusual fatigue. This may be because women are more prone to the development of microvascular disease, a narrowing of the smaller arteries that supply blood to the heart.
Stable angina is a predictable pattern of chest discomfort with a similar degree of severity and classic precipitating factors occurring either recently or over several months. It maintains a constant threshold in severity and relief over time. The corresponding lesion is usually a stable, fixed atherosclerotic lesion that is flow-limiting only when myocardial metabolic demands reach a threshold.
Unstable angina refers to angina of recent onset, intensifying in nature with a lower level of exertion or occurring nocturnally without immediate relief by rest or with nitroglycerin. The underlying coronary lesion in this emergent situation is usually a critical stenosis with either acute coronary vasospasm or thrombus formation, resulting in intermittent or permanent vessel occlusion. Unstable angina requires prompt evaluation and treatment. Coronary angiography is the gold standard of diagnosis, which will locate and assess the severity of coronary artery lesions. The adverse outcomes of unstable angina include MI and sudden death due to arrhythmias such as ventricular tachycardia or ventricular fibrillation (Merck Manual for Healthcare Professionals, 2013).
Variant angina, originally described by Prinzmetal, refers to chest pain occurring almost exclusively at rest, not precipitated by physical exertion or emotional stress. It is associated with ECG ST-segment elevation. It has been demonstrated that variant angina is related to coronary artery spasm with subsequent myocardial ischemia. In addition, it may be associated with MI, ventricular tachycardia, and ventricular fibrillation as well as sudden death (Keller & Lemberg, 2004).
Angina can also be a symptom of coronary microvascular disease, also called cardiac syndrome X or nonobstructive CAD. This is a heart disease that affects the heart’s smallest coronary arteries and is more likely to affect women than men.
Silent myocardial ischemia can occur in patients with angina at rest, unstable angina, and chronic stable angina. It has been clearly established that patients who are at high risk of CAD can be evaluated by stress testing for silent but significant ECG ST-segment changes (Cohn, Fox, & Daly, 2003). Prognostically, this may be the only way to limit sudden death from MI as well as to identify and avoid an initial or subsequent MI.
Other conditions that should be included in the differential diagnosis of angina include common painful esophageal reflux, achalasia, esophageal spasm, peptic ulcer, and biliary colic (Table 8.2). Chest wall discomfort with localized pain, tenderness, and swelling of the costal cartilages, described in 1931 as Tietze’s syndrome, is a common additional differential diagnosis. Cervical radiculitis, chest wall spasm, and severe pulmonary hypertension associated with chest pain on exertion may all simulate anginal pain. Pulmonary embolism, acute pericarditis, mitral valve prolapse, and herpes zoster can all be included in the differential diagnosis. Although all of these can in most cases be readily distinguished from angina by a detailed history and a comprehensive physical examination, the primary care provider must ensure that chronic CAD does not simultaneously exist with noncardiac disease.
Physical Examination
Patients with chronic CAD and stable angina are often found to have an entirely normal physical examination. The physical examination may reveal signs of other conditions such as heart failure, valvular heart disease, or vascular disease. In some patients, the BP may be chronically elevated. It is the responsibility of the primary care provider to perform a diligent and complete physical examination on all patients. The primary care provider can often identify useful clues to the diagnosis of CAD and identify patients who are at the highest risk of this disease.
Differential Diagnosis of Chest Pain |
CARDIAC |
Coronary artery disease Aortic stenosis Pericarditis |
PULMONARY |
Pleuritis Pneumonia Tracheobronchitis Pneumothorax Tumor |
VASCULAR |
Aortic dissection Pulmonary embolism Abdominal aortic aneurysm |
GASTROINTESTINAL |
Esophageal spasm Esophageal perforation Esophageal reflux Biliary colic Peptic ulcer disease Pancreatitis |
MUSCULOSKELETAL |
Costochondritis Cervical radiculopathy Subacromial bursitis |
OTHER |
Herpes zoster Fibrocystic breast disease |
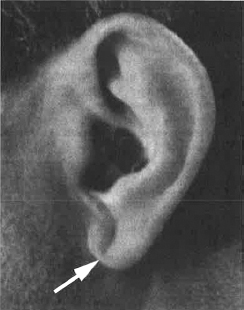
A diagonal crease in each earlobe (Frank’s sign; Figure 8.2) was originally described as a marker of coronary disease with a moderate sensitivity (approximately 48%) and specificity (approximately 88%). This sign has been subsequently associated with other cardiac risk factors, such as diabetes, hypertension, hypercholesterolemia, tobacco use, and obesity as well as cerebral infarction (Zapata-Wainberg & Vivancos, 2013).
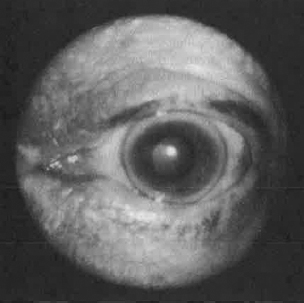
Ophthalmologic examination may reveal a corneal arcus (Figure 8.3). The size of the corneal arcus positively correlates with age, levels of total cholesterol, and levels of LDL (Chen et al., 2009). The skin around the eyelid may demonstrate xanthelasma, which may be promoted by increased levels of triglycerides and a relative deficiency of HDLs. The retina may reveal arteriolar atherosclerotic hypertensive or diabetic changes.
Of utmost importance in the general physical examination is to check all arterial pulses and search for abnormalities in the venous system. The association between peripheral arterial disease and CAD is well documented. This is not confined only to patients with symptomatic disease, clinically overt peripheral arterial disease, or carotid artery disease, but is also seen in asymptomatic, ultrasonically proven, hemodynamically significant obstructive arterial disease. Examination of the patient’s venous system can reveal a history of a previous venous CABG.
FIGURE 8.2
Photograph of diagonal earlobe crease, which may have a correlation with coronary artery disease.
The cardiac examination may be most helpful during an episode of symptomatic angina because ischemia may produce transient left ventricular dysfunction with a third heart sound and bibasilar pulmonary rales. A softened mitral component of the first heart sound and paradoxical splitting of the second heart sound may occur during an anginal attack. A mid-systolic click followed by a late systolic murmur may occur in patients with CAD related to transient papillary muscle dysfunction with alteration in the alignment of the papillary muscles.
 DIAGNOSTIC STUDIES
DIAGNOSTIC STUDIES
Noninvasive Testing
ELECTROCARDIOGRAPHY
Patients with chronic stable angina have normal ECG tracings at rest, but they may have severe CAD. However, the 12-lead ECG during a provoked episode of substernal chest pain or during an attack of angina may reveal downward or horizontal sloping depression of the ST-segment, T-wave peaking, or T-wave inversion. These ECG changes accompanied by chest discomfort may signal the possibility of coronary stenosis. ECG findings that indicate a poorer prognosis include evidence of a prior MI with Q waves in multiple leads, as well as the presence of a left bundle branch block, a second- or third-degree atrioventricular block, ventricular arrhythmias, or left ventricular hypertrophy (Fihn et al., 2012).
There are no specific guidelines for ECG follow-up, particularly if the initial ECG is normal. Naturally, there are exceptions. Poorly controlled BP, a family history of premature cardiovascular death, high lipids, or glucose abnormalities, for example, warrant repetition of ECGs on an individual basis (Townsend, 2001).
EXERCISE ECG
Exercise treadmill ECG has been the most studied noninvasive test for the evaluation of CAD in both men and women. Stress testing increases overall cardiac work and heart rate. This increased work results in an increase in myocardial oxygen demand and a subsequent requirement for increased oxygen delivery via an increase in coronary blood flow. If narrowed or obstructed lesions prevent the required increase in coronary blood flow, chest pain or ECG changes may arise. In both sexes, the exercise ECG is most accurate in patients who are able to exercise to 85% of their maximal predicted heart rates (Banerjee, Newman, Van den Bruel, & Heneghan, 2012). However, this goal can be modified based on the characteristics of the patient being evaluated. Younger patients with a low risk of CAD will have a much more sensitive result should they reach their maximal heart rate target or exercise to exhaustion. Older, high-risk patients, in contrast, may reveal clinically meaningful information at lower cardiac workloads. Ischemia is also dependent on the severity of obstruction, as coronary artery stenoses of <70% are frequently undetected by functional testing (Fihn et al., 2012).
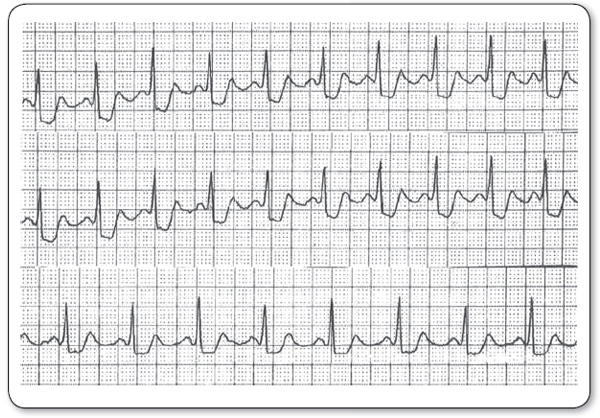
Depression or downsloping of the ST-segment 0.08 seconds after the J point (the junction between the end of the QRS complex and the beginning of the ST-segment) on the ECG is the typical ischemic change sought as a positive result (Figure 8.4). Current guidelines recommend a diagnostic end point of ≥1 mm horizontal or downsloping ST depression as the criterion for positivity (Fihn et al., 2012; http://circ.ahajournals.org/content/126/25/3097.full.pdf+html). If this end point is set low (i.e., 0.5 mm), the test will be more sensitive but less specific, whereas if the end point is set high (i.e., 2 mm), the result will be less sensitive but more specific. If typical chest discomfort occurs during the test associated with an ST depression of more than 1 mm, the predictive value for the detection of CAD is 90% (Gibbons et al., 2002). ST depressions of more than 2 mm in the setting of typical chest discomfort are virtually diagnostic of CAD.
FIGURE 8.4
Downsloping of the ST-segment 0.08 seconds after the J point of the electrocardiogram is the typical ischemic change sought as a “positive” result during exercise stress testing.
The referring provider must be mindful of the fact that the results of exercise stress testing are likewise affected by the patient’s pretest probability of having disease. In men with classic angina pectoris or a previous MI, a positive test result will accurately predict CAD about 85% of the time (Gibbons et al., 2002; http://circ.ahajournals.org/content/106/14/1883.long). Similarly, the results of exercise stress testing in women may be less sensitive than those obtained in men, given the lower prevalence of CAD in women younger than 70 years. Asymptomatic, low-risk patients also have a significant false-positive rate that may range as high as 60% (Gibbons et al., 2002).
In general, exercise stress testing is used for prognosis, diagnosis, and assessment of the effectiveness of therapy (Table 8.3). Diagnostic interpretations must be performed with pretest probabilities of disease for each patient kept in mind. As a prognostic test, a submaximal ECG stress test can identify patients at high risk after MI. Likewise, a positive result after minimal exertion is frequently indicative of three-vessel disease and should prompt angiography if appropriate. Documentation of improved exercise capacity and ischemic ECG changes after medical, interventional, or surgical therapy can guide future treatment plans.
Indications and Contraindications to Exercise Stress Testing |
INDICATIONS | CONTRAINDICATIONS |
Diagnose the etiology of chest pain Assess prognosis Assess the need for angiography Evaluate functional capacity Assess degree of ischemia after myocardial infarction Assess adequacy of revascularization after coronary artery bypass grafting Ascertain the effects of medical, interventional, or surgical management of coronary artery disease | Recent onset of unstable angina Uncontrolled hypertension Severe decompensated heart failure Significant ventricular arrhythmias Severe obstructive valvular disease Associated cardiopulmonary conditions, including severe pulmonary hypertension, myocarditis, pericarditis conditions, recent pulmonary embolism, rapid atrial fibrillation |
Contraindications to exercise stress testing are listed in Table 8.3 and include any underlying condition that might preclude an appropriate increase in cardiac output after exercise. Recent onset of unstable angina or MI is an absolute contraindication to performing a maximal stress test, but submaximal exercise testing for prognostic assessment, activity prescription, or assessment of medical therapy can be performed as early as 4 to 6 days after MI with relative safety (Gibbons et al., 2002). A symptom-limited exercise test can be performed about 3 to 6 weeks post-MI.
Indications to Add Imaging to Standard ECG Stress Testing |
Left bundle branch block
Paced ventricular rhythm
Resting ST-segment depressions
Left ventricular hypertrophy
Digoxin use
Preexcitation syndromes
ECG, electrocardiogram.
An exercise stress test will be the initial functional test ordered for most patients. Certain ECG features (Table 8.4) limit the identification of ischemia by exercise testing (Fihn et al., 2012). Patients with these features should undergo stress testing with imaging either with echocardiography or a radionuclide study. Patients who are unable to achieve an adequate exercise level should undergo pharmacological stress testing.
STRESS ECHOCARDIOGRAPHY
While exercise ECG is the most widely used technique for the diagnosis of suspected or known CAD, only 40% of patients can perform a diagnostic exercise test (Senior et al., 2005). ST-T wave changes occur late in the ECG test and therefore ischemia and detection of CAD are less accurate if patients do not achieve at least 85% of their maximal predicted heart rate. Stress echocardiography may be performed in conjunction with treadmill or bicycle exercise. The diagnostic end point is new or worsening wall motion abnormalities or changes in global left ventricular function as a result of exercise (Fihn et al., 2012). In patients who are unable to exercise, pharmacological agents such as dobutamine or dipyridamole may be used in a stress echocardiogram test.
Stress echocardiography is a reliable technique not only for the diagnosis of suspected CAD but also for the accurate risk stratification of patients with suspected and established CAD (Senior et al., 2005). Advances in image acquisition, digital display, and the development of contrast imaging have improved the reliability of this test. Stress echocardiography now allows simultaneous assessment of myocardial function and perfusion, and tissue Doppler imaging allows quantitation of wall motion. Stress echocardiography is commonly used in patients with heart failure both for assessing the etiology of heart failure and to evaluate and determine the extent of ischemic disease. Diagnostic sensitivity of stress echocardiography ranges from 70% to 85% for exercise and 85% to 90% for a pharmacological test, with specificity ranging from 77% to 89% for exercise and 79% to 90% for a pharmacological test (Fihn et al., 2012). Ready availability and reliability make stress echocardiography a cost-effective technique for the assessment of CAD (Senior et al., 2005). However, diagnostic accuracy can be influenced by the technique of the echosonographer and a patient’s physical characteristics that may reduce image quality.
RADIONUCLIDE STUDIES
ECG-gated myocardial perfusion single-proton emission computed tomography (SPECT, also known as gated SPECT) is another imaging modality for assessing myocardial viability and can improve upon the accuracy of the diagnosis of CAD over exercise ECG alone. Technetium-99m (Tc-99m) agents and thallium-201 are the common radioisotopes used in SPECT. These agents are rapidly extracted by viable myocardium. During exercise, the radioisotope is preferentially distributed to coronary artery territories that are maximally dilated. Stenotic coronary arteries are not capable of dilating in response to exercise and blood flow is therefore stolen from these areas. This appears as a perfusion defect on a nuclear scan. At rest, the maximally dilated coronary arteries resume normal flow patterns and blood again flows through the stenotic lesions. In follow-up scans, the area of radioisotope defect will now appear perfused, and this reversible perfusion defect signifies an area of viable myocardium with disadvantaged flow (ischemia). An irreversible perfusion defect is suggestive of a fixed obstructive lesion with no underlying viable myocardium (i.e., myocardial scar or infarct). Hibernating myocardium is the result of severe coronary artery stenosis that causes chronic hypoperfusion and ischemia at rest. Being able to distinguish between hibernating myocardium and scar is essential, as the function can be restored to a hypoperfusion area of hibernating myocardium by revascularization. Revascularization of an area of myocardial scar will not improve cardiac function (Fathala, 2011). Diagnostic sensitivity of SPECT ranges from 82% to 88% for exercise and 88% to 91% for a pharmacological test, with specificity ranging from 70% to 88% for exercise and 75% to 90% for a pharmacological test (Fihn et al., 2012).
SPECT has the ability to allow the nuclear test reader to observe myocardial contractions in segments with apparent fixed perfusion defects in order to discern attenuation artifacts from true perfusion abnormalities. The ability of SPECT to provide measurement of left ventricular ejection fraction, segmental wall motion, and absolute left ventricular volumes also adds to the prognostic information that can be derived from a SPECT study.
The use of either radionuclide or echocardiographic imaging can increase the prognostic value of the stress test and either should be used when the results are likely to add diagnostic information. Conditions such as mitral valve prolapse, left ventricular hypertrophy, and left bundle branch block, as well as digitalis use, may cause repolarization abnormalities that obscure stress-induced ECG changes. In such situations, imaging can significantly increase the detection of inducible ischemia over ECG stress testing alone. SPECT is preferred in patients with a left bundle branch block or paced rhythm, though stress echocardiogram is also an option, but less well studied. Other indications for imaging studies include the risk stratification of patients before major noncoronary surgery and in patients after MI. Radionuclide imaging studies are useful in the assessment of myocardial viability in patients undergoing revascularization risk stratification (Askew & Chareonthaitawee, 2013; Fihn et al., 2012). The choice of imaging modality is based on availability, expertise, cost, patient’s body habitus, patient preference, or the need for concomitant assessment of hemodynamics or valvular function.
PHARMACOLOGICAL STRESS TESTS
Pharmacological stress tests are inexpensive and safe diagnostic tools with excellent specificity and good sensitivity. They are primarily used in patients who cannot exercise for ECG or imaging stress tests. The choice of a pharmacological agent, either a vasodilator or a positive inotrope, is dependent on patient characteristics, the type of stress test, and provider preference.
Vasodilating agents include dipyridamole, adenosine, and regadenoson. They work by increasing intracellular cyclic adenosine monophosphate levels and inhibiting the formation of thromboxane A2, a potent vasoconstrictor. When injected, they act as coronary vasodilators. Stenotic coronary lesions are already maximally dilated and cannot respond to vasodilating agents. Nonstenotic coronary arteries maintain their ability to vasodilate, and preferential flow down these newly dilated vessels results in coronary steal from the stenotic arteries. Infusion of a vasodilating agent is contraindicated in patients with bronchospastic pulmonary disease, those with significant hypotension, or patients with sick sinus syndrome and high-degree heart block. Theophylline and caffeine should be avoided for 48 to 72 hours prior to a pharmacological stress test with a vasodilator, as these agents may decrease the effectiveness of the vasodilating agent.
Dobutamine is a synthetic beta-1 and beta-2 agonist with positive inotropic effects. Dobutamine causes myocardial ischemia primarily through a marked increase in myocardial oxygen demand resulting from increased heart rate, BP, and myocardial contractility. The increase in coronary blood flow achieved is comparable to that during physical exercise but slightly less than that with vasodilating agents. Dobutamine stress testing is an appropriate alternative in patients who cannot exercise and who have contraindications to vasodilating agents. Dobutamine stress testing causes ventricular ectopy in 40% to 50% of patients; a recent history of tachyarrhythmias is a relative contraindication.
From a meta-analysis of 82 studies (Kim, Kwok, Heagerty, & Redberg, 2001), it was concluded that the sensitivity of dipyridamole SPECT imaging (89%) was higher than that of dipyridamole echocardiography (80%), but the specificity of dipyridamole SPECT imaging (65%) was lower than that of dipyridamole echocardiography (93%). The sensitivity of dobutamine echocardiography (80%) was similar to that of dobutamine SPECT imaging (82%), but dobutamine echocardiography had a higher specificity (84%) than dobutamine SPECT imaging (75%).
Either vasodilating or positive inotropic agents may be used in stress testing in lieu of treadmill exercise, and they achieve similar sensitivity (Fihn et al., 2012). When coupled with radionuclide imaging, CAD detection is similar to that of exercise radionuclide testing, so pharmacological stress testing is a powerful tool in patients who cannot exercise. Pharmacological stress echocardiography has also been used in the diagnosis and management of CAD with a prognostic value similar to radionuclide testing (Sicari et al., 2008).
CT CORONARY ANGIOGRAM
The 64-multislice CT coronary angiogram is a noninvasive, fast, and accurate procedure that captures three-dimensional (3-D) images of the heart. The results of multiple meta-analyses and clinical trials report a sensitivity ranging from 93% to 97% and a specificity ranging from 80% to 90% for detecting obstructive CAD (Fihn et al., 2012). The coronary artery scan can reveal plaque (CAD) many years before it can be found by physical examination, ECG, or stress testing. If plaque is present, early treatments can help to prevent MI or the need for revascularization. The 64-multislice CT coronary angiogram helps to identify CAD in individuals at risk of the disease and can be useful in patients not diagnosed with CAD but whose symptoms suggest that the condition could be present, in those who have an inconclusive stress test, or those who are having chest pain and have risk factors for developing CAD.
The 64-multislice CT coronary angiogram is an easy and reliable tool for comprehensive in vivo diagnosis of myocardial bridging. Myocardial bridging is a rare cause of chest pain in a subgroup of younger patients with less prevalence of hyperlipidemia and more prevalence of cardiomyopathy than patients with significant atherosclerotic CAD (de Agustín et al., 2012). A myocardial bridge is a band of heart muscle that lies on top of a coronary artery; so part of a coronary artery dips into and underneath the heart muscle and then comes back out again. The band of muscle that lies on top of the coronary artery is called a “bridge,” and this is how the condition gets its name.
As previously stated, CAD is the leading cause of mortality in the United States. While invasive angiography is regarded as the gold standard for the diagnosis of CAD, invasive angiography is not without its potential risks, although, according to ACC/AHA guidelines, the risk of major complications is <2% (Scanlon et al., 1999). At the same time, lingering concerns about radiation safety in CT coronary angiogram exist. It has been unclear what risk of cancer is attributable to CT coronary angiogram scans. A 128-slice CT scan is now available. The 128-slice CT coronary angiogram is ideal because of its high accuracy in the assessment of significant coronary stenosis and significant reduction in radiation exposure (Ghadri et al., 2012).
Cardiac catheterization and selective coronary arteriography is the gold standard for visualization of the in vivo morphological details of the coronary arteries (Fihn et al., 2012). Access to the coronary circulation is usually achieved through the femoral artery. In patients with severe peripheral vascular disease, an alternative arterial entry using the radial or brachial artery can be used. Each coronary ostium is then selectively cannulated and an angiogram of each vessel is taken in several standard views.
Coronary angiography can detect as little as 20% narrowing of the main coronary arteries, as well as a critical stenosis of 70% or greater (de Bono, 1993). The cardiac catheterization can also assess hemodynamic measurements, left ventricular function, valve surface area, and valvular gradients.
A broad list of indications for cardiac catheterization and coronary angiography is shown in Table 8.5. Relative contraindications include uncontrolled ventricular arrhythmias, uncorrected metabolic disturbances, decompensated heart failure, uncontrolled hypertension, elevated prothrombin time, severe allergy to contrast agents, and severe renal insufficiency.
The incidence of complications of coronary arteriography is low but is directly related to the skill and experience of the interventional cardiologist as well as to the severity of the cardiac condition (see the section under Nonpharmacological Interventions). The risk of major complications is under 2% for all patients (Scanlon et al., 1999). The incidence of death is 0.11% (Encyclopedia of Surgery, 2013). Cardiologists regard an ejection fraction reading <35%, age >60 years, or advanced valvular disease as markers for increased risk of complications.