Chapter 13 Considerations in the preoperative assessment of paediatric patients
Simon Moore
SUMMARY
This chapter will cover the following:
• introduction and definitions
• anatomical and physiological considerations
• psychological considerations
• practicalities of anaesthetising children
• postoperative care.
INTRODUCTION
When assessing a paediatric patient preoperatively it is important to be aware of the considerable differences that exist in terms of anatomy, physiology, pharmacology and psychology, when compared to the adult patient. The younger the patient, the more pronounced these differences will be. It is also important to note that children vary enormously in weight, size, shape, intellectual ability and emotional response.
Special consideration will need to be given to neonates, ex-premature infants and those with congenital abnormalities. Ideally these patients will be dealt with in a tertiary paediatric centre, but may present to a district general hospital in an emergency situation. The approach to the elective and emergency situation follows the same broad pattern but time constraints will inevitably leave less time for thorough psychological preparation when inevitably the focus is on the adequacy of airway, breathing and circulation.
Definitions
• Neonates are babies less than 44 weeks post-conception.
• Infants are less than one year old.
• A child is between one and 12 years old.
• An adolescent is between 13 and 19 years old.
• A premature infant is an infant born before 37 weeks post-conception.
• Low birth weight is a birth weight of less than 2500g.
Anatomical and physiological considerations
A. Airway and respiratory system
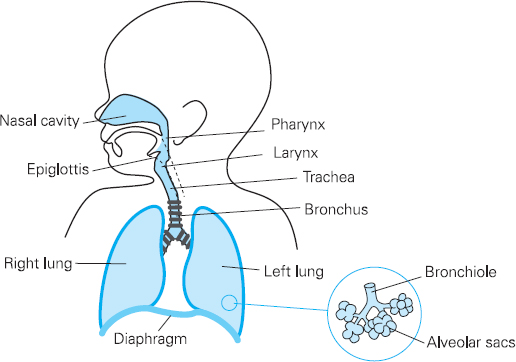
Figure 13.1 Paediatric airway and respiratory system
Anatomy
The anatomy of the airway and respiratory system has some of the most pronounced differences between adult and child. The head is relatively large and the neck is short. Coupled with this, the tongue is large and can obstruct the pharynx, leading to airway obstruction. An oral airway may be needed to aid ventilation.
Neonates are mainly nose breathers so any decrease in the already narrow nasal passages (50% of total airway resistance) from secretions or oedema can have a significant effect on gas flow and the work of breathing. Careful consideration should be given to any child with coryzal symptoms (see preoperative visit section below).
The larynx is anterior and cephalad (C3-C4). Positioning for intubation will therefore be different from that of an adult, as a ‘sniffing the morning air’ position in a neonate or infant will impair visualisation of the glottis. The use of a head ring to stabilise the head, or a roll under the shoulders, aids intubation. The epiglottis is long, stiff and U-shaped. A straight blade is often easier for intubation, as it lifts the epiglottis away from the cords. It must be remembered that the posterior part of the epiglottis is innervated by the vagus nerve; therefore intubation with a straight blade can lead to a bradycardia.
The paediatric airway is narrowest at the cricoid cartilage, just below the vocal cords. Here, the trachea is lined with pseudo-stratified ciliated epithelium, loosely bound to the underlying alveolar tissue. Any trauma which results in oedema will impede gas flow. The trachea is short (5cm in the neonate) so endotracheal tubes have to be placed and secured carefully to avoid an endo-bronchial intubation. The tracheal cartilages are soft and easily compressed and can collapse if breathing is attempted against an obstructed airway. The right main bronchus is larger than the left and less acutely angled at origin. This invariably leads to right-sided endobronchial intubation (see Figure 13.2).
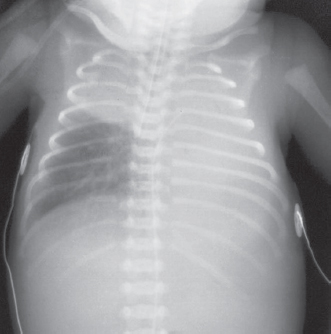
Figure 13.2 Malpositioned endotracheal tube – tube has passed along the right main bronchus distal to the origin of the right upper lobe bronchus with subsequent collapse of the left lung and right upper lobe.
Reproduced with kind permission from: Dr Richard Hopkins, Consultant Radiologist and Editor of Hopkins, R., Peden, C. & Ghandi, S.: Radiology for anaesthesia and intensive care (2nd edition), Cambridge Medical
The ribs are horizontal rather than bucket handle as in adults. Ventilation is therefore mainly diagphragmatic. Bulky viscera or gastrointestinal obstruction, leading to gas-filled bowel, can impede diaphragmatic excursion, leading to respiratory embarrassment.
The chest wall is very compliant and is pulled inward by the lungs, decreasing functional residual capacity (FRC). Closing capacity (CC) exceeds FRC during normal respiration in neonates, infants and children up to the age of six to eight years. Applying continuous positive airway pressure (CPAP) intraoperatively improves oxygenation and reduces the work of breathing.
At birth the tracheo-bronchial tree is developed to the terminal bronchioles. The alveoli are thick walled and number 20 million. By the age of six, the number of alveoli has increased to 300 million. Further growth is seen as an increase in the size of the alveoli and airways.
Physiology
Oxygen consumption is markedly raised in paediatric patients, and can exceed 6ml/kg/min in infants, i.e. double the adult value of 3–4ml/kg/min. To meet this increased demand, alveolar minute ventilation is increased via an increase in respiratory rate (tidal volume is the same as in adults at 7ml/kg). This leaves infants vulnerable to hypoxaemia in a very short space of time if there is any upper airway obstruction.
The maturation of neuronal respiratory control is related to post-conceptual age rather than postnatal age. Both hypoxic and hypercapnic drives are not well developed in neonates and infants. Importantly, hypoxia and hypercapnia cause respiratory depression in this age group. Postoperative apnoea occurs commonly in the preterm neonate.
Respiration is diaphragmatic, sinusoidal and continuous in nature rather than periodic, as seen in older children and adults.
B. Cardiovascular and haematology system
A basic understanding of the foetal circulation and the circulatory changes that occur at birth is important in understanding the response to conditions of severe hypoxia, hypercapnia, hypothermia and acidosis that can occur over the first few weeks of life. Essentially in these stressful conditions reversion to a foetal ‘transitional’ circulation can occur if conditions are not improved.
In the foetus little blood flow occurs to the pulmonary circulation, as the resistance to flow is high in the collapsed lungs. Blood flows from right to left atria via the patent foramen ovale and from the pulmonary artery to the aorta via the ductus arteriosus. This arrangement results in relatively oxygen-rich blood reaching the brain. At birth as the lungs become aerated, pulmonary vascular resistance falls, both directly and as a result of increasing oxygen tension, leading to an increase in pulmonary blood flow through the lungs to the left atrium. Pressure within the left atrium becomes higher than that in the right, and the foramen ovale begins to close. Systemic vascular resistance rises as blood flow to the placenta ceases. Aortic pressure rises above that in the pulmonary artery and blood flow in the ductus arteriosus reverses. The increased arterial oxygen tension leads to constriction of the ductus arteriosus over the first few days. In hypoxic conditions, these flow changes can reverse and potentially lead to a spiralling hypoxaemic decline.
Table 13.1 Cardiovascular and haematological physiological variables
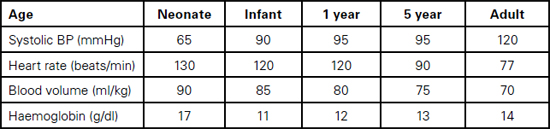
Foetal haemoglobin HbF, which makes up 70–90% of haemoglobin at birth, is not able to deliver oxygen as efficiently to tissues as normal haemoglobin because it holds on to oxygen more avidly (less 2,3 Bisphosphoglycerate). The high haemoglobin level (13–19g/dl, depending on the degree of placental transfusion) and high cardiac output compensate for this. Within three months, the levels of HbF drop to around 15% and HbA predominates. Haemoglobin levels drop over three to six months to 9–12g/dl as the increase in circulating volume increases more rapidly than the bone marrow activity (physiological anaemia).
Vitamin K dependent clotting factors (II, VII, IX, X) and platelet function are deficient in the first few months. Vitamin K is given at birth to prevent haemorrhagic disease of the newborn.
C. Renal system and fluid management
Renal vascular resistance is high immediately after birth. It decreases rapidly and is low by two weeks. Glomerular filtration rate and renal blood flow are therefore low in the neonate and increase steadily throughout childhood, reaching adult levels around the age of two. Concentrating capacity of the infant kidney is less than that of an adult. Maximum urine osmolality is 600–700mOsm/kg, compared to 1200mOsm/kg in the adult. Despite the low glomerular filtration rate, infant kidneys can handle large water loads because of their low concentrating capacity. After a free water load infants can excrete a markedly dilute urine of 50mOsm/kg compared to a maximally dilute urine of 70–100mOsm/kg in adults.
Table 13.2 Glomerular filtration rates by age
| Age | ml/min/1.73 m2 * |
| 1–2 days | 20–25 |
| 1 week | 35–40 |
| 3 months | 55–60 |
| Adult | 120 |
| * Glomerular filtration rate is indexed to body surface area; this is inexact because of the relatively high body surface area of neonates and infants. | |
The loop of Henle is short and this affects the kidneys’ ability to retain sodium via the ascending limb of the loop. Even with a low glomerular filtration rate, a net loss of sodium can occur. Carbonic anhydrase is present in the proximal tubules at birth, but is scanty in the distal tubules. There are also lower concentrations of buffering ions and this, in combination with the decreased carbonic anhydrase, decreases the kidneys’ ability to excrete acid.
Dehydration is poorly tolerated as children have large insensible losses due to the large surface area to weight ratio. There is a larger proportion of extra cellular fluid in children – 40% body weight compared to 20% in adults.
Fluid management in the paediatric surgical patient can be divided into three categories: deficit therapy, maintenance therapy and replacement therapy, all aimed at maintaining a urine output of 1–2ml/kg/hour. As with any prescribed treatment, fluid therapy should be monitored and adjusted according to response. Plasma electrolytes and glucose should be checked at least every 24 hours (more frequently in neonates).
Deficit therapy
Deficit therapy is the management of fluid and electrolyte losses prior to the patient’s admission. This is a major consideration for patients presenting as emergencies following trauma or infective episodes of diarrhoea and vomiting.
Fluid deficit can be estimated from the history, examination and electrolyte evaluation. Examination includes an evaluation of the eyes and fontanelles (may be sunken), skin colour, turgor and temperature, character and rate of peripheral pulse, respiratory rate, mental state and capillary refill time. Initial fluid boluses of 20ml/kg crystalloid or 10ml/kg colloid followed by reassessment may be necessary. A fall in blood pressure is a sign of uncompensated hypovolaemic shock.
Maintenance therapy
Maintenance fluid requirements are calculated as shown below, on an hourly basis depending on body weight. They replace the fluid the child would normally have been drinking.
• 4ml/kg for the first 10kg body weight
• 2ml/kg for the next 10kg body weight
• 1ml/kg for each kg above 20kg body weight.
After most minor to moderate surgery, children will go back to drinking normally fairly quickly.
Replacement therapy
After major surgery or trauma, blood may need to be replaced. Also ‘third space’ losses will need replacing. This refers to fluid lost from the circulation, in the form of oedema, into the bowel and by evaporation. This is normally calculated as 7–10ml/kg/hour of surgery. Children, like adults, generally show a stress response to surgery, with a rise in blood glucose even if no glucose-containing fluid is given. There is an increase in antidiuretic hormone (ADH) secretion, leading to retention of water by the kidneys.
Most fluid is replaced as Hartmann’s solution with colloid or blood used as necessary. Use of 0.9% sodium chloride and dextrose solutions depends on the requirements of the child. The routine prescription of hypotonic solutions such as 4% glucose/0.18% saline in the perioperative period is no longer acceptable practice with its attendant risk of hyponatraemia.
D. Gastrointestinal system
Hepatic enzyme systems are initially immature. Barbiturates and opioids, for example, have a longer duration of action and neonates are more prone to jaundice (glucuronyl transferase system is poorly developed). Carbohydrate reserves are low in neonates and they are more prone to hypoglycaemia. Regular blood glucose measurements should be performed and hypoglycaemia treated with 10% glucose. Hyperglycaemia is usually iatrogenic and can result in neurological damage
E. Temperature control
Neonates and infants have a large surface area to weight ratio, which increases heat loss. They also have minimal subcutaneous fat and poorly developed shivering, sweating and vasoconstriction mechanisms. Brown fat, which requires more oxygen for metabolism, is used for non-shivering thermogenesis in the neonate.
Heat loss during anaesthesia is mainly via radiation but can also be attributed to convection, conduction and evaporation. Low body temperature increases the duration of action of drugs, decreases platelet function and increases the risk of infection. It also causes respiratory depression, acidosis and decreased cardiac function. Children having prolonged intra-abdominal surgery, with a large surface area exposed to the operating theatre environment, are particularly vulnerable.
F. Central nervous system
The neonatal blood brain barrier is poorly formed. Drugs such as barbiturates, opioids, antibiotics and bilirubin cross the blood brain barrier easily, causing a prolonged and variable duration of action. The minimum aveolar concentration (MAC) of volatile agents is lower in neonates due to a relatively higher proportion of fat in the neonatal brain. MAC then peaks at one year (50% higher than adult values) decreasing thereafter, reaching adult levels by puberty.
Neonates can appreciate pain and this is associated with increased heart rate, blood pressure and a neuro-endocrine response. There is also evidence to suggest that infants who experience painful stimuli have an increased sensitivity to pain when older, possibly as a result of changes in the central nervous system.
Psychology
Children vary in their intellectual ability and emotional response. Some knowledge of child development will be helpful in understanding their fears and behaviour and will enable the perioperative team to take an appropriate approach to the child’s surgery. Hospitalisation and medical procedures have profound emotional consequences for infants and children and behavioural disturbances can occur long after the event. The most important determinant of the impact of hospitalisation is age.
• Infants under six months are generally not upset by separation from parents.
• Children of six months to four years are much more upset by hospitalisations, primarily because of separation from family and familiar surroundings.
• School age children are more concerned with the procedure itself.
Factors other than age also influence the child’s emotional response, such as length of stay, type of procedure and parental reaction. ‘[B]eing unable to choose parents for your patients, you must make do with those who come with the child’.1
Therefore for elective procedures adequate time must be set aside for psychological preparation for both children and parents. This may involve a visit to the hospital, play simulation, video presentations and age-specific literature for the child to read. Long-term behavioural benefits have been demonstrated in patients who participate in such preoperative preparation. A previous theatre experience may have gone badly and these children may benefit from a more thorough preoperative psychological preparation.
Even with a thorough approach it is still sometimes difficult to predict how children will cope once in theatre. Some who appear quite calm and understanding of the process on the ward preoperatively may react badly once in the anaesthetic room.
Practicalities for anaesthetising children
A. Preoperative visit
Use this important time to develop rapport and gain the confidence of both child and parents. Answer any questions in simple language and avoid wearing a white coat. Question the child directly, if possible, but include the parents in any discussion. Explain the approach to induction of anaesthesia and inform and obtain consent for the use of suppositories. Discuss postoperative pain management and any local anaesthetic procedures that will be performed. Take a thorough history and examine the child as necessary. Review the case notes, observation charts and any investigations performed. Prescribe premedication and local anaesthetic creams if necessary. Ensure adequate consent has been obtained. ‘At age 16 a young person can be treated as an adult and can be presumed to have capacity’1 to consent to any treatment. A child under 16 may also have capacity to decide on any treatment if they have an understanding of what is involved. For further details on consent, the General Medical Council (GMC) has produced guidance, available at: http://www.gmc-uk.org/guidance/current/library/consent.asp
Anaesthetic and medical history
The following items should be addressed when taking a history:
i. Problems with previous anaesthetics including postoperative nausea and vomiting (PONV), poor pain relief, difficult venous access.
ii. Family history to exclude malignant hyperthermia (MH) and Suxamethonium apnoea.
iii. Current or previous medical problems. If a significant history is obtained, communication with other healthcare professionals may be necessary to establish the current disease status. If a child presents with signs of an upper respiratory tract infection (URTI) try to establish whether this is the start or end of the cold. If at the start postpone surgery for two weeks. If the child is considered postviral, surgery is probably safe. Any child with a productive cough, pyrexia or constitutional illness should be postponed.
iv. Neonatal history.
v. Current medications.
vi. Allergies.
vii. Recent immunisations: surgery should be avoided for one week after DTP and Hib and two weeks after MMR.
viii. Check for the presence of loose teeth.
ix. Ensure the child adequately fasted:
• 6 hours for solids and milk if more than 12 months of age
• 4 hours for breast milk and formula feeds if less than 12 months of age
• 2 hours for unlimited clear fluids (as this decreases gastric acidity and volume).
There is an increased incidence of nausea and vomiting with long fasting periods.
Examination
Conduct a physical examination as appropriate, concentrating on the airway and cardiorespiratory systems. Children must be weighed, as the child’s weight determines the dosage of drug to be administered. In the absence of actual weight, the following equations will give an approximate estimated weight to allow clinicians to calculate appropriate drug dosages especially in emergency situations:
• For children aged 3–12 months: weight (in kg) = [age (in months) + 9] / 2
• For children aged 1–6 yrs: weight (in kg) = [age (in years) + 4] x 2
Investigations
The following investigations should be considered for the following groups of patients:
• haemoglobin – large expected blood loss, premature infants, systemic disease, congenital heart disease
• electrolytes – renal or metabolic disease, intravenous fluids, dehydration
• chest X-ray – active respiratory disease, scoliosis, congenital heart disease.
Pre-medication and topical anaesthetics
• Analgesics – Drugs such as paracetamol, ibuprofen or codeine phosphate, given more than a half an hour preoperatively, are useful for shorter procedures, as they will have had time to reach an effective therapeutic level for the intra- and postoperative period.
• Sedation – Sedation should not be given as a matter of routine as it is unpleasant to take, difficult to time, can fail or cause agitation and may delay recovery and discharge. However, it may be necessary in an excessively anxious child or a child with a previous unpleasant anaesthetic or hospital experience. Oral midazolam (0.5mg/kg), given 30 minutes preoperatively, is widely prescribed. Adding Calpol or a sweetener, such as fruit juice, helps reduce the bitter taste. Alternative agents include oral ketamine (5–6mg/kg) (with or without midazolam), clonidine (4mg/kg), trimeprazine (2mg/kg), promethazine (1mg/kg), chloral hydrate (50mg/kg to a maximum of 1g) and temazepam (0.5–1mg/kg)(for older children).
• Anticholinergic drugs – Vagal blocking drugs (atropine, hyoscine, glycopyrrolate) are no longer given routinely but atropine should be immediately available to use if it becomes necessary. Atropine is the preferred anticholinergic in children, as it is most effective at blocking the cardiac vagus nerve (0.02mg/kg). The same dose can be given orally 90 minutes preop or intramuscular 30 minutes preop. It can also be given through the tracheal tube in the same dose diluted in saline to 2ml in an emergency.
• Topical anaesthetics – Local anaesthetic cream is applied to identifiable veins on those children for whom an intravenous induction is planned. EMLA cream is a eutectic mixture of 5% lignocaine and 5% prilocaine in a 1:1 ratio. It needs to be applied one hour prior to cannulation and lasts 30–60 mins. It also produces vasoconstriction and there is a risk of methaemoglobinaemia (prilocaine) in children less than one year old. Ametop is a 4% gel formulation of the ester local anaesthetic, amethocaine, and has a shorter onset time of 45 minutes. It is less readily absorbed across mucous membranes and does not cause methaemoglobinaemia. It also has the advantage of a prolonged duration of action (four hours) after the cream is removed. It causes local erythema and vasodilatation, making cannulation easier. It is more likely, however to cause allergic reactions and should be removed after 90 minutes.
B. Preparation for anaesthesia
As with any anaesthetic, the preparation starts prior to the preoperative visit when the anaesthetist finds out what patients are on the operating list. Occasionally at a district general hospital, there may be paediatric patients who are not on a dedicated paediatric list. If this is the case, the child must be operated on ‘first on the list’, so the starvation period is not extended.
The anaesthetic plan will have been formulated with the child and his or her parents during the preoperative visit. During this visit it is a good idea to decide which parent, if either, will accompany the child to the anaesthetic room. If one of the parents is present for induction, a member of staff must be available to escort the parent back to the ward from the anaesthetic room. This can be a very stressful experience for the parent.
Prior to the child arriving in the anaesthetic room, drug doses should be calculated according to the weight of the child. Emergency drugs, such as atropine and suxamethonium, should be drawn up in dilutions that are familiar.
All equipment should be ready and checked. Airways, facemasks, laryngeal masks, endotracheal tubes, laryngoscopes and blades and breathing circuits appropriate to the age and weight of the patient. Alternate sizes of all of the above should also be readily available.
Most anaesthetists use uncuffed tubes until the age of approximately eight years to reduce the risk of subglottic stenosis (the narrowest part of the paediatric airway is at the level of the cricoid cartilage). A small leak should be present; if the leak is too big, ventilation will be compromised. Cuffed tubes are available down to 5.5mm. Tube sizes and lengths can be estimated as follows:
- Tube size = age/4 + 4.5
- Tube length = age/2 + 12
These are guides to sizes and tubes should be checked for a leak, and length should be checked by auscultating the chest.
Laryngeal mask sizes relate to weight as follows:
- size 1 – up to 5kg
- size 1.5 – 5–10kg
- size 2 – 10–20kg
- size 2.5 – 20–30kg
- size 3 – over 30kg
Endotracheal tubes and laryngeal mask airways should be secured with tape fixed to the less mobile maxilla.
As discussed earlier, children lose heat rapidly. The theatre should be at an appropriate temperature. Optimal ambient temperature to prevent heat loss is 32°C for neonates and 28°C for adolescents and adults. Warming devices (mattress, forced air warmers and fluids) and padding should also be prepared. Use of a circle breathing system will also help to conserve heat.
The plan should be made known to the operating department practitioner or anaesthetic nurse.
C. Induction of anaesthesia
1) Intravenous
Placing an intravenous cannula has been made easier with the advent of local anaesthetic creams (see pages 262–3). Veins on the back of the hand or inner wrist are the easiest to find. Alternatives include the long saphenous vein, other veins on the dorsum of the foot and antecubital fossa in older children. The child can be placed on the parent’s lap or on the trolley and the hand concealed from the child’s view. The arm may be squeezed gently by an anaesthetic assistant or by the anaesthetist with the non-cannulating hand. The child should be distracted in some way or asked to cough during the cannulation attempt. Intravenous induction can be undertaken with propofol, thiopentone or ketamine.
2. Inhalational
Sevoflurane is the current volatile agent of choice due to its non-irritant nature, rapid onset and relative haemodynamic stability. It can be given either with a high initial concentration (8%) or increased gradually, usually with the addition of nitrous oxide. Halothane is an alternative volatile agent which may still be used in some centres. A variety of techniques can be used in performing an inhalational induction and it is important to develop a fluid approach depending on how the child and parents react. The child may be placed supine on the trolley or on the parent’s lap. They may wish to hold the mask themselves or have the parent hold the mask. A clear, flavoured mask is less intimidating than a black mask. A cupped hand method may also be used. Warn parents that the child’s head will become floppy and explain there is an excitatory phase accompanied by abnormal movements. Once anaesthesia is achieved, a skilled assistant will need to maintain the child’s airway while intravenous access is obtained. A correctly sized (size from the corner of the mouth to the angle of the jaw) oropharyngeal airway may be used. Airways should not be inverted during insertion, as there is a risk of damage to the palate.
D. Maintenance
Techniques using nitrous oxide, oxygen and either sevoflurane, isoflurane or desflurane are accepted methods of maintaining anaesthesia in children. Sevoflurane and desflurane have a more rapid offset than isoflurane but do show more emergence agitation than the latter.
Total intravenous anaesthesia (TIVA) has advantages for patients at high risk of vomiting, such as, children undergoing strabismus surgery or surgery to the middle ear. Maintenance of anaesthesia with propofol has been shown to have significant anti-emetic effect.
The pharmacokinetics of propofol in children differs from adults. A three compartment model is applicable to children with a volume of distribution that is 50% larger than adults. Clearance is up to twice that of adults. Clinically this means that a higher initial bolus dose is needed to reach a given plasma concentration and increased infusion rates to maintain steady state plasma concentrations.
Neonates and infants are more sensitive than adults to non-depolarising muscle relaxants, though initial doses are similar, due to a larger volume of distribution. Duration of action may be prolonged in neonates due to decreased glomerular filtration rate and hepatic clearance. Larger doses of suxamethonium (2mg/kg for infants) are similarly required due to the larger volume of distribution.
Postoperative care
Children should be recovered in a designated paediatric recovery area (which can be within the adult recovery room), with easy access to appropriate equipment and correctly sized blood pressure cuffs and pulse oximeters. Staff should have received training in recovery of paediatric patients and provide one-to-one care. The area should be kept warm to prevent hypothermia and provision should be made for a parent or carer to rejoin their children as soon as they are awake. The usual standards of handover and discharge criteria apply.
Postoperative pain relief is provided by a standard multimodal approach with paracetamol and NSAIDs widely prescribed for minor cases. The rectal loading dose should be at least 30mg/kg, followed by 15mg/kg six hourly. Aspirin should not be used in children under the age of 12 years because of the association with Reye’s syndrome. For moderate to severe pain, codeine or oromorph can be added. Morphine infusions, nurse-controlled analgesia or patient-controlled analgesia pumps can also be used if closely supervised by appropriately trained ward staff.
Local anaesthetic techniques including wound infiltration and specific regional blocks should always be considered as part of the multimodal approach to pain relief (see below).
Postoperative nausea and vomiting can be appropriately managed with the use of combinations of 5-HT3 antagonists with low dose dexamethasone. Children at particular risk, such as those undergoing tonsillectomy or squint surgery, should receive prophylactic ondansetron (0.1mg/kg IV) at induction of anaesthesia.
Regional anaesthesia
Most children would not tolerate surgery under a regional technique alone. However, such a technique is a very useful adjunct to general anaesthesia for postoperative pain relief. Tetracaine eye drops can be used after strabismus surgery and lidocaine ointment is useful after circumcision.
Wound infiltration with local anaesthetic is easy to perform and very effective. Some older children will tolerate procedures such as naevi removal with local infiltration alone.
Peripheral nerve blocks are also used as an adjunct to general anaesthesia. A penile nerve block is used after circumcision, minor hypospadias repair and other penile procedures. Ilioinguinal–iliohypogastric nerve blocks are effective analgesia for inguinal herniotomy and for the groin incision for orchidopexy.
Epidurals in children are beyond the remit of the district general hospital (DGH). There are not enough staff trained on the paediatric ward in a DGH to safely look after epidurals. Surgery requiring that level of postoperative pain relief should be done at a tertiary centre.
Caudal extradural anaesthesia (CEA) is a widely used, simple, safe and effective technique for intraoperative and postoperative analgesia. It is especially suited to the paediatric population due to the relative ease with which landmarks can be identified, the higher dermatomal block that can be achieved and the greater spread of local anaesthetic, due to less tightly packed epidural fat. The potential for lower limb weakness with caudal anaesthesia can be minimised with weaker local anaesthetic solutions. One of the drawbacks of the single shot caudal is that effective analgesia only lasts for a few hours. Addition of NMDA antagonists, such as ketamine 0.5mg/kg or alpha-2 agonists, such as clonidine 1-2µg/kg can double or even quadruple the duration of the analgesia.
REFERENCE
1. Mellish, R. (1969). Preparation of a child for hospitalization and surgery. Paediatric Clinics of North America 16 (3): 543–53.
FURTHER READING
A.R. Aitkenhead and R.M. Jones (1996). Clinical Anaesthesia. Oxford: Churchill Livingstone.
K.G. Allman and I.H. Wilson (2006). Oxford Handbook of Anaesthesia (2nd edn). Oxford: Oxford University Press. 758–87.
L.J. Brennan (1999). Modern day-case anaesthesia for children. British Journal of Anaesthesia 83: 91–103.
Chidanande-Swamy, M. and Mallikarjum, D. (2004). Applied aspects of anatomy and physiology of relevance to paediatric anaesthesia. Indian Journal of Anaesthesia 48(5): 333–9.
General Medical Council (2008). Consent: Patients and Doctors Making Decisions Together. London: GMC.
R. Hopkins, C. Peden and S. Gandhi (2003). Radiology for Anaesthesia and Intensive Care. London: Greenwich Medical Media.
L. Rusy and E. Usaleva (1998). ‘Paediatric Anaesthesia Review Update’. Anaesthesia issue 8. Available at: http://www.nda.ox.ac.uk/wfsa/html/u08/u08_003.html
C.L. Snyder, T.L. Spilde and H. Rice (2008). Fluid Management for the Paediatric Surgical Patient. Available at:
http://emedicine.medscape.com/article/936511-overview (updated 24 January 2008).
D.J. Steward and J. Lerman (1975). Manual of Pediatric Anesthesia (5th edn). London: Churchill Livingston.
S.M. Yentis, N.P. Hirsch and G.B. Smith (2009). Anaesthesia and Intensive Care A to Z: An Encyclopedia of Principles and Practice. (4th edn) Oxford: Churchill Livingstone.
INTERNET RESOURCES
www.gmc-uk.org/guidance/ethical_guidance/children_guidance_appendix_1.asp

Full access? Get Clinical Tree





