FIGURE 26-1. Schematic diagram of the gate control theory as proposed by Melzack and Wall in 1965. The theory suggests that sensory input from the periphery converges on a group of secondary neurons (T) in the spinal cord, which, in turn, convey sensory input to higher centers in the brain. This transmission of sensory input from first-order to second-order neuron within the spinal cord serves as the “Gate” for sensory input and is modified by interneurons located within the substantia gelatinosa. If nonnociceptive sensory input traveling via large nerve fibers converges on the same secondary neurons, this nonpainful input closes the “Gate” preventing transmission of painful nociceptive input. SCS was developed based on the gate control theory with the idea that stimulating nonnociceptive fibers electrically might close the gate and reduce pain perception. While the gate control theory has proven overly simplistic, the concept that nonnociceptive nerve traffic can modify pain perception has proven true.
Over the past five decades, the implantation of SCS systems has evolved in many areas, including patient selection and technology as well as a focus on improved outcomes and improved health care utilization. The increase in physician implantation has been driven by a sharpened focus on pain treatment by the medical community and society, a desire to reduce the need for chronic opioid medications, and the goal of improving function. With the increase in the number of patients being offered SCS therapy, there has been a focus on improving technology by both physician innovators and medical device manufacturers.
The initial systems used for SCS had a single lead with one anode and one cathode. These rudimentary devices also had limited ability to be programmed and consisted of nothing more than an on/off mechanism triggered by a handheld magnet. Evolution of newer devices and implant techniques has improved effectiveness by using multiple electrode arrays with a variety of cathode and anode combinations, multiple leads to increase paraesthesia coverage, and smaller generators to improve comfort and acceptance. In recent years, rechargeable batteries, percutaneously placed paddle leads, and new patient pain mapping software have been introduced and show promise for improving the usefulness of SCS even further. Because of variances in approval criteria, some products, such as those used to introduce percutaneous paddle leads and new paddle constructs, are available outside the United States prior to being available in the United States. It is important for physicians to be up-to-date on evolving technologies since many of these new technologies will impact practice by improving the success of stimulation and reducing the rate of complications.
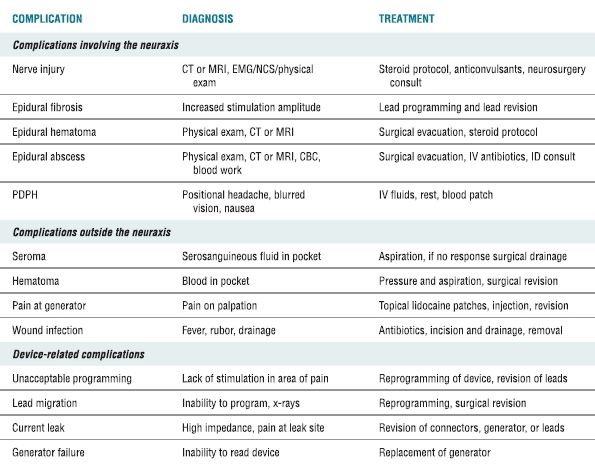
Early retrospective case series3 and prospective, observational trials4 established the usefulness of SCS for treating chronic pain after prior lumbar surgery, particularly chronic radicular pain. Recently, SCS proved to provide superior pain relief, based on visual analog scales and global patient satisfaction, when compared to repeat operation in those patients having recurrent or persistent pain following prior lumbar fusion.5 Critically important in this era of cost-based medicine, the available evidence suggests that SCS results in a net savings to the health care system when compared with conservative pain treatment techniques.6 SCS in conjunction with a structured physical therapy program also leads to better functional improvement in patients with complex regional pain syndrome than physical therapy alone.7 SCS is now established as an effective technique for treating many types of chronic pain (Box 26-1). Like any surgical technique, particularly those requiring placement of an indwelling device, complications will arise in a small number of patients. It is important to understand the potential complications when offering this therapy to patients. Complications can be broken down into those relating to the neuraxis, those involving the device and its components, and those occurring in the tissues outside the spine. In order to have success in managing these complications, the physician must understand the complications that can occur, how to recognize them, and the best corrective measures for each (Table 26-1).
BOX 26-1 Established Indications for Spinal Cord Stimulation
 Chronic lumbar radiculopathy
Chronic lumbar radiculopathy
 Chronic cervical radiculopathy
Chronic cervical radiculopathy
 Neuropathic limb pain secondary to spinal disease prior to initial surgery
Neuropathic limb pain secondary to spinal disease prior to initial surgery
 Failed cervical surgery syndrome with radicular component
Failed cervical surgery syndrome with radicular component
 Failed back surgery syndrome (including persistent pain in the back and/or the extremities)
Failed back surgery syndrome (including persistent pain in the back and/or the extremities)
 Primary axial back pain prior to intrathecal drug administration
Primary axial back pain prior to intrathecal drug administration
 Spinal stenosis
Spinal stenosis
 Postherpetic neuralgia
Postherpetic neuralgia
 Peripheral nerve injury
Peripheral nerve injury
 Brachial plexopathy
Brachial plexopathy
 Radiation neuritis
Radiation neuritis
 Complex Regional Pain Syndrome (Types I and II)
Complex Regional Pain Syndrome (Types I and II)
 Raynaud’s syndrome
Raynaud’s syndrome
 Ischemic pain of the extremity
Ischemic pain of the extremity
 Visceral pain syndromes including abdominal pain from pancreatitis
Visceral pain syndromes including abdominal pain from pancreatitis
 Visceral pain syndromes including pelvic pain from interstitial cystitis
Visceral pain syndromes including pelvic pain from interstitial cystitis
 Angina pectoris
Angina pectoris
 Diabetic peripheral neuropathy and other neuropathies of the upper and lower extremities
Diabetic peripheral neuropathy and other neuropathies of the upper and lower extremities
TABLE 26.1 Complications Associated with SCS, Their Diagnosis, and Treatment
 SCOPE OF THE PROBLEM
SCOPE OF THE PROBLEM
The incidence of complications associated with spinal cord stimulator placement has been reported in frequencies ranging from 0% to 81%, depending on the author and significance of the adverse event reported. In a systematic analysis focusing on higher quality studies, the mean complication rate was 34% when reviewing multiple data pools. In this same analysis, the need to do a surgical revision was 23%, while those requiring explant was 11%. Life-threatening or serious complications occurred <1% of the time.8 Cameron performed an extensive review of literature encompassing 3,679 patients and reported a complication rate of 36%. In another long-term analysis of 102 implanted patients, the surgical revision rate was 32% over a 10-year follow-up period.9 Burchiel and colleagues found a 17% revision rate with patients implanted for 1 year or more.10 Kumar11 found lead complication rates to be 5.3%, epidural fibrosis rates of 19%, and infection rates of 2.7%. Furthermore, Kumar et al.,12 in a later study, reported a frequency of stimulators requiring revision of approximately 25% to 33%, and of those requiring revision, 85% remained satisfied with the results.
The most serious complications involve the spine and associated structures. Device complications are much more common and require more frequent surgical attention. The complications surrounding programming and device computer malfunction are a frustration to the patient and clinician, but are minor when compared to the potentially life-threatening problems that involve spinal structures. Complications of structures outside the neuraxis vary in severity and can be as simple as pain at an incision or generator or as complicated as serious wound infections leading to sepsis.13–16 Kemler17 reported that biologic complications from SCS typically occur within 3 months of implant, while device complications occur later, but typically still within the first 2 years after implantation.
 DEFINITION, INCIDENCE, AND DIAGNOSIS OF COMPLICATIONS ASSOCIATED WITH SPINAL CORD STIMULATION
DEFINITION, INCIDENCE, AND DIAGNOSIS OF COMPLICATIONS ASSOCIATED WITH SPINAL CORD STIMULATION
Complications Involving the Neuraxis
Among the most serious and urgent complication of SCS is the development of an epidural hematoma. The incidence of this complication is low, and no reliable estimate of the incidence can be made from the existing literature, although it is thought to be <1%. While unproven, because SCS involves placing a large introducer needle into the epidural space and then threading an electrode many levels cephalad within the spinal canal, it seems likely that the incidence of epidural hematoma formation would be somewhat higher than that during single shot or continuous epidural analgesia. Exact numbers are not available. The development of an epidural hematoma can lead to paralysis if not treated in a timely fashion.18 The patient may complain of numbness developing in the immediate postoperative period. This numbness may be accompanied by severe back or leg pain. Weakness in the postoperative period should be seen as a red flag for suspicion of an epidural hematoma. Risk factors for developing this complication include use of anticoagulants, aspirin, nonsteroidal anti-inflammatory drugs, or other drugs affecting platelet function. Readers are directed to the American Society of Regional Anesthesia guidelines for regional anesthesia in the patient receiving anticoagulation therapy.19 Other risks include difficult lead placement with multiple lead passes, the need to place a surgical lead requiring laminectomy, and reoperation in an area previously implanted. This complication is more common with surgical instrumentation requiring extensive bone removal.8 Diagnosis of an epidural hematoma in this setting requires computed tomography (CT). Magnetic resonance imaging (MRI) can be obtained if the device has been removed. While MRI has been done in patients with implants in place without difficulty, MRI has not been approved by the Food and Drug Administration for use in patients with indwelling SCS leads20–22 over concerns that the strong magnetic field may induce inductive heating within the epidural lead and resultant tissue injury.
Another serious and potentially life-threatening complication is infection of the spinal structures. Possible infections include epidural abscess, discitis, and meningitis. The incidence of such infections is small, and no data exist on the exact percentage, but most authors have quoted an incidence of <1 in a 1,000.23 Epidural abscess is the most common of these complications, and considerations are similar to those discussed for epidural analgesia (see chapter on infectious complications). Meglio et al.24 described a case of an intradural and epidural abscess with associated paraplegia in a patient with a recently explanted spinal cord stimulator. Discitis and meningitis often result as a progression of the epidural abscess into surrounding tissues. The patient with a developing epidural abscess often complains of severe pain in the area of the lead implant. An elevated temperature above 101 degrees Fahrenheit may also be suggestive of abscess formation and bacteremia. The development of radiating pain in a dermatomal or nondermatomal fashion may indicate progression of the abscess. Risk factors for abscess formation include an immunocompromised state, uncontrolled diabetes mellitus, chronic dialysis, a history of organ transplant with ongoing immunosuppressive medications, history of chronic skin infections, history of methicillin-resistant staphylococcal aureus infection, and localized skin infection or breakdown at the surgery site. Diagnosis of an epidural abscess or discitis can be confirmed by CT or using MRI, once the device has been removed. The diagnosis of bacterial meningitis diagnosis is best confirmed by prompt cerebral spinal fluid (CSF) analysis.23
Neurologic injury of the spinal cord or nerve roots is a potential risk of SCS. Direct trauma to neural structures caused by the epidural needle used to introduce the electrode is the most common reported mechanism for this complication, and other mechanisms include injury by lead placement, lead removal, and traction on nerves while placing a surgical laminectomy lead. In many reported cases of nerve injury, the patient was under general anesthesia or deep sedation at the time of injury and could not respond with complaints of a paraesthesia at the time of neural contact. Meyer et al.25 described such a case where an intradural percutaneous lead was discovered at C2 on postoperative CT in a patient that was undergoing a planned IPG replacement (but unplanned lead replacement) under general anesthesia. The patient developed quadriparesis due to direct injury to the spinal cord.25 Evaluation should be guided by the symptoms reported by the patient. Imaging studies are unlikely to reveal any abnormalities following isolated injury to a single nerve root, even in the patient reporting ongoing painful dysesthesiae. In contrast, any patient who develops signs or symptoms suggesting injury to the spinal cord should undergo immediate imaging with CT (in the event the epidural electrode remains in place) or MRI (if the lead was removed). MRI is far more sensitive in delineating subtle injury to the substance of the spinal cord itself. In some cases, direct visualization of the cord or nerve tissue by open surgical examination is required to confirm an injury, remove a paddle lead, or repair a dural tear. Electromyograms and nerve conduction studies can also be helpful in defining the location and extent of nerve injury, but may not show an abnormality for several weeks following injury (see Chapter on Nerve Injury).26
A more frequent but less-worrisome neuraxial complication is that of inadvertent dural puncture. In a study involving patients with complex regional pain syndrome reported by Kemlar, 11% of 36 patients in the randomized clinical trial had evidence of postdural puncture headache (PDPH).27 Other authors have noted similar rates of this complication. Factors that may predispose to this complication are previous surgery in the area of needle placement, calcified ligaments, obesity, and patient movement. Technique may also play a role with a higher rate of dural puncture occurring with the midline approach, steep angle of needle placement (above 45 degrees), and the use of a needle to introduce a retrograde lead. An extremely rare, but more severe complication is a dural tear with subsequent chronic CSF leak. This can lead to chronic positional headache, nausea, tinnitus, and malaise. The introduction of a foreign body into the epidural space can result in scarring of the epidural tissues. Lenarson et al.28 reported a histologically confirmed foreign body reaction causing symptomatic compression of the cervical spinal cord secondary to a percutaneous epidural SCS lead. Epidural fibrosis is a predictable result of lead placement into the epidural space, although it has been described as a complication.29 Scarring begins shortly after lead introduction and, as stimulation is dependent on tissue impedance, may adversely alter the therapeutic stimulation characteristics. Kumar et al.30 describe “system tolerance” as loss of analgesic effect despite, continued concordant paraesthesia coverage, partly attributed to the epidural scarring.
Patients who require SCS often have significant pathologic changes of the bony spine, and these abnormalities can progress in the years following placement of an implanted system. In cases where a lead is successfully placed, the development of spinal stenosis may result in compression of the spinal structures, and the presence of an electrode in the epidural space may well contribute to the degree of central canal stenosis, resulting in new radicular symptoms or signs of myelopathy.31 Diagnosis of spinal stenosis in the patient with an indwelling lead is made by history, physical exam, and eventually by CT myelography most often in consultation with a neurosurgeon.
Complications Involving Nonspinal Tissues
Infection involving the implanted pulse generator pocket or the paraspinous course of the electrode can result in the need to remove the device. The incidence of wound infection ranges from 0% to 4.5%, although some have reported higher rates of infection.32 Diagnosis of infection is most often straightforward, with erythema, tenderness, and drainage of purulent material from the sites of surgical placement of the lead and/or IPG. In deep-seated infections or early in the course of infection, laboratory values may help define the presence and severity of infection: elevated white blood count, elevated C-reactive protein, and elevated erythrocyte sedimentation rate. These tests are nonspecific markers of inflammation and may indicate a postopera-tive atelectasis, blood clot, or urinary tract infection. An elevated white blood cell count, with a left shift in the differential cell count toward neutrophils along with abnormality in either of the other two studies should prompt increased vigilance by the implanting physician. Infections may occur in the pocket, lead placement incision, or tunneled subcutaneous tissue. Infections may involve only the superficial tissues or may be widespread, extending from the pocket to the epidural space.
The noninfectious buildup of serosanguineous fluid in the pocket can lead to a seroma that may impede the ability of the wound to heal, can cause pain, and can lead to poor patient satisfaction even if the device is providing effective pain relief. Seroma is diagnosed when normal laboratory values are present, wound Gram stain and culture are negative, fever is absent, yet an erythematous, swollen, fluctuant wound is present. Diagnosis is confirmed by aspiration of straw-colored fluid that does not show bacteria on micro-scopic analysis or subsequent culture. Adherence to meticulous surgical technique can reduce the risk of seroma, but cannot eliminate it entirely.33
The Centers for Disease Control and Prevention release guideline statements regarding surgical site in-fection perioperative prevention strategies, including commentary on surgeon, patient, and environmental factors.34 Of the factors listed in Box 26-2, appropriate hand preparation by the surgeon, a sterile operating theater environment, type of skin preparatory solution (chlorhexidine is preferred to povidone iodine), use of preoperative antibiotics administered 30–minutes prior to incision, and wound irrigation are all sup-ported by conclusive scientific study. Preoperative bathing, the use of antibiotic irrigation, occlusive drapes, dressing removal time, antibacterial ointments, or postoperative antibiotics have only anecdotal evidence and may contribute to microbial antibiotic resistance.35 Bleeding can occur in the wound of the generator or lead placement area. The bleeding can range from superficial bruising to large volume hema-toma requiring treatment.36 Bedder et al.37 commented on the use of bacterial ointment after closure, de-scribing that routine antibiotic ointment may increase antibiotic resistance, increase allergy to the antim-icrobial, and increase susceptibility to skin necrosis.
BOX 26-2 Avoiding Infection and Seroma Formation
 Screen the patient preoperatively for signs or symptoms of skin, dental, urinary, or pulmonary infection.
Screen the patient preoperatively for signs or symptoms of skin, dental, urinary, or pulmonary infection.
 If signs or symptoms suggestive of infection are present, obtain a preoperative white blood cell count with differen-tial.
If signs or symptoms suggestive of infection are present, obtain a preoperative white blood cell count with differen-tial.
 Administer prophylactic antibiotics 30 min before start of device placement.
Administer prophylactic antibiotics 30 min before start of device placement.
 Recommend that the patient bathe with chlorhexidine or similar agent the morning of surgery.
Recommend that the patient bathe with chlorhexidine or similar agent the morning of surgery.
 If the patient has a history of MSRA infection or colonization, use intranasal mupirocin or similar antibiotic for 72 h prior to surgery (consider consultation with an infectious disease specialist for guidance regarding eradication of MRSA–colonization).
If the patient has a history of MSRA infection or colonization, use intranasal mupirocin or similar antibiotic for 72 h prior to surgery (consider consultation with an infectious disease specialist for guidance regarding eradication of MRSA–colonization).
 Review that the antibiotic covers common microbes detected in your facility.
Review that the antibiotic covers common microbes detected in your facility.
 Prepare the skin using at least six successive applications of chlorhexidine that extend least 24 inches outside of your suspected surgical field.
Prepare the skin using at least six successive applications of chlorhexidine that extend least 24 inches outside of your suspected surgical field.
 Use chlorhexidine gluconate surgical preparation solution.
Use chlorhexidine gluconate surgical preparation solution.
 If hair removal is required to prepare the operative field, clipping is suggested over shaving. If shaving is required, it should be performed immediately before the surgical skin preparation.
If hair removal is required to prepare the operative field, clipping is suggested over shaving. If shaving is required, it should be performed immediately before the surgical skin preparation.
 Drape at least 12 inches outside of your projected operative field.
Drape at least 12 inches outside of your projected operative field.
 Avoid direct contact with the C-arm once it is moved into the lateral position.
Avoid direct contact with the C-arm once it is moved into the lateral position.
 Adhere to meticulous sterile technique.
Adhere to meticulous sterile technique.
 Adhere to meticulous gentle surgical technique during tissue dissection, avoiding aggressive blunt dissection.
Adhere to meticulous gentle surgical technique during tissue dissection, avoiding aggressive blunt dissection.
 Irrigate the wounds under low pressure with copious amounts of saline prior to closure.
Irrigate the wounds under low pressure with copious amounts of saline prior to closure.
 Ensure complete hemostasis within the pocket and paraspinous incisions.
Ensure complete hemostasis within the pocket and paraspinous incisions.
 Ensure all dead space is closed and a layered closure is performed.
Ensure all dead space is closed and a layered closure is performed.
 Ensure that all skin edges are even and no tension is on the skin during closing.
Ensure that all skin edges are even and no tension is on the skin during closing.
 Consider the use antibiotic ointment on the incisions prior to placing the sterile dressing.
Consider the use antibiotic ointment on the incisions prior to placing the sterile dressing.

Full access? Get Clinical Tree


