FIGURE 32-1. Conventional RF lesion. A 10-cm RF cannula with a 5-mm active tip is immersed in egg white and a lesion is carried out at 80°C for 90 seconds. The size of the lesion is maximal near the mid-portion of the active tip, with little coagulation at the tip of the needle. Thus, for optimal application of conventional RF treatment the shaft of the needle’s active tip must be placed adjacent to the target. The size of the lesion is near maximal by 60 seconds of treatment, changing little in size thereafter. (Reproduced with permission from Rathmell JP. Atlas of Image-guided Intervention in Regional Anesthesia and Pain Medicine. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2012.)
Pulsed RF uses brief “pulses” of high-voltage RF-range (~300 kHz) electrical current that produce the same voltage fluctuations in the region of treatment that occur during conventional RF treatment but without heating to a temperature at which tissue coagulates (Box 32-1). The conceptual appeal of a minimally invasive nondestructive technique that is useful in treating chronic pain of any sort is compelling. In clinical practice, there has been a mass migration to the use of pulsed RF despite little data to support efficacy of this new technique. The modality has great appeal, specifically because it is not neurodestructive. With conventional RF, the thermal lesion occasionally leads to worsening pain and even new onset of neuropathic pain.6 A small retrospective case series7 and extensive clinical experience among practitioners suggest that pulsed RF results in neither increased pain nor any risk of neuropathic pain and that it is very well tolerated by patients from treatment through recovery. This technique is in need of further study to validate its usefulness.
BOX 32-1 How Is “Radiofrequency” Energy Used to Treat Pain?
RF generators produce high-frequency voltage fluctuations in the range of 300 to 500 kHz, the medium frequency range of the electromagnetic spectrum used in amplitude modulated broadcasting.
Conventional (thermal) RF treatment utilizes a continuous output of RF voltage fluctuations that produces movement of ions within the tissue adjacent to the uninsulated tip of the needle or “cannula.” Movement of ions leads to heating within the tissue. The temperature at the tip of the cannula is measured continuously during treatment using a small wire probe inserted in the cannula, the thermocouple. The power output of the generator is adjusted to maintain a constant temperature at the tip of the cannula, typically 80CC. The size of the uninsulated or “active” tip of the cannula (typically ranging from 2 to 10 mm) and the temperature maintained during treatment determine the size of the lesion that results. Tissue coagulation begins above about 45°C; thus, conventional RF treatment has also been termed “neurolysis,” “neurotomy,” or “ablation.”
Pulsed RF treatment uses brief “pulses” of the same range (~300 kHz) of RF voltage fluctuations, using cannula and thermocouple identical to those used during conventional RF treatment. The energy is applied intermittently, typically in pulses of energy lasting 20 to 30 milliseconds and repeated twice each second (2 Hz). The power output and the duration of the pulses are varied automatically by the RF generator to assure that the tip temperature does not rise above a set temperature point, most often 42°C, to assure that no tissue coagulation occurs. It has been demonstrated that applying such pulses of energy adjacent to specific neural structures can produce changes in gene expression within neurons in the dorsal horn of the spinal cord.8 However, how or if these changes in gene expression relate to reduction in pain and the clinical utility of pulsed RF treatment awaits further validation.9
 DEFINITION
DEFINITION
Conventional wisdom holds that the usefulness of RF stems from the ability to produce a small lesion of precise dimensions in a specific anatomic location.10 Pulsed RF produces the same voltage fluctuations in the region of treatment as conventional RF but without tissue coagulation. Complications associated with RF treatment fall into two broad categories: those that arise during placement of the RF cannula and those that result from neural destruction during conventional RF neurotomy. As may occur with any technique that requires needle placement, direct injury to neural or vascular structures in the vicinity of treatment can occur. Both conventional and pulsed RF treatment are associated with similar risk of injury during needle placement. Conventional RF neurotomy using a heat lesion that produces neural destruction is also associated with the sequelae of neural injury that follow placement of lesions. Even with proper technique, conventional RF lesions are associated with sensory loss and onset of neuropathic pain in a subset of patients. The frequency and severity of these neural changes vary with the specific site of treatment and are most common after intracranial RF lesioning of the trigeminal ganglion. Injury to adjacent nerves can also occur, and the frequency of these complications is ininimized by the proper use of sensory and motor stimulation before lesioning.
 RADIOFREQUENCY TREATMENT FOR TRIGEMINAL NEURALGIA
RADIOFREQUENCY TREATMENT FOR TRIGEMINAL NEURALGIA
Scope
Trigeminal neuralgia (tic douloureux) is a common idiopatnic form of neuropathic pain that presents with paroxysms of pain involving one or more division of the trigeminal nerve (cranial nerve V). The disorder typically strikes those in the sixth decade of fife, is of sudden onset without a precipitating event, and has a 2:1 predominance in females (Table 32-1).11 Patients report episodic and severe pain involving one or more branches of the trigeminal nerve. The most commonly affected branches are the second and third divisions together, followed closely by involvement of either the second or third division alone. Those affected often describe a small area that “triggers” paroxysms of pain. Perhaps, pressure on an incisor or slight touch to the margins of the lip precipitates lancinating pains along the entire course of the involved division of the trigeminal nerve. The pain typically occurs in brief paroxysms lasting just seconds at a time, but may escalate to a frequency of many episodes daily.12 The underlying etiology remains unknown, but experts have hypothesized that second-order neurons within the trigeminal ganglion become sensitized, and following sensory input from the trigger area seizure-like hyperactivity is triggered in adjacent sensory neurons and is manifested as pain. Recurrent trauma due to pulsatile compression of the ganglion caused by the small branch vessels from the adjacent carotid siphon has been postulated as the underlying cause of neural injury that leads to trigeminal neuralgia,13,14 and from this hypothesis treatment via posterior craniotomy and microvascular decompression has evolved as a successful treatment.15
TABLE 32.1 Characteristics of 154 Consecutive Patients with Trigeminal Neuralgia Treated with RF Rhizotomy
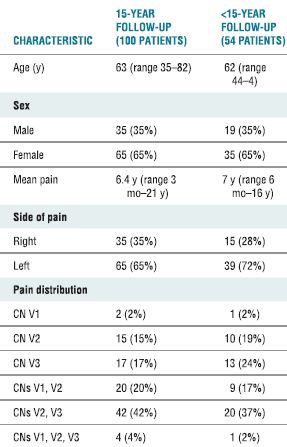
CN, cranial nerve.
Adapted from Taha JM, Tew JM Jr, Buncher CR. A prospective 15-year follow up of 154 consecutive patients with trigeminal neuralgia treated by percutaneous stereotactic radiofrequency thermal rhizotomy. J Neurosurg 1995;83:989–993.
Upon initial presentation, patients should undergo a thorough neurologic examination to exclude other causes that might precipitate similar symptoms, such as an intracranial tumor or demyelinating disease. In the majority of cases, no underlying etiology can be clearly identified, and treatment begins with antiepileptic drugs. Carbamazepine has long been the drug of first choice and is successful in controlling the pain in the majority of patients.16 Indeed, early practitioners often regarded response to carbamazepine as diagnostic for this disorder. At the current time, owing to the appearance of blood dyscrasias and hepatotoxicity in a minority of those receiving carbamazepine, newer antiepileptic drugs (including lamotrigine, oxcarbazepine, gabapentin, pregabalin, and duloxetine) are often used prior to treatment with carbamazepine.
Invariably, a proportion of patients will continue to experience debilitating pain despite drug treatment or their pain will recur and become refractory to pharmacologic management, and this group will seek further treatment. A wide range of neuroablative techniques have been developed, all aimed at destroying a small number of cells within the affected area of the trigeminal ganglion and thereby eliminating the neuronal excitability.17 Available techniques range from chemical destruction with glycerol to focused external-beam radiotherapy (radiosurgery), and among these neuroablative techniques the most widely used has been percutaneous RF ablation of the trigeminal ganglion (Table 32-2).17 The use of high-resolution magnetic resonance imaging (MRI) and radiosurgery has rapidly evolved in recent years, and many experts place this treatment modality foremost among available treatment options.18 Choosing among the available treatment techniques can be difficult, but most experts currently recommend microvascular decompression via a posterior fossa approach for those patients without coexisting medical illness that would make the risk of this major surgery unacceptable.17 All of the available neuroablative techniques will produce some degree of sensory and/or motor loss. Microvascular decompression is the only treatment approach associated with a high success rate without creating neurologic deficits and without the need for ongoing pharmacologic therapy. However, because this is a major intracranial surgery, there is a small but significant risk of major neurologic injury and death.
TABLE 32.2 Results of Percutaneous Techniques and Posterior Fossa Exploration for Patients Treated for Trigeminal Neuralgia
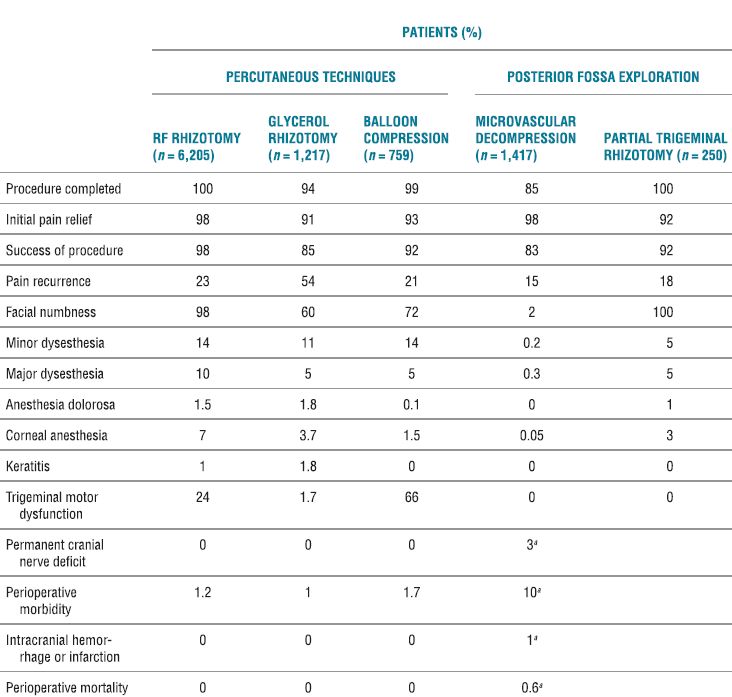
aCombined values for microvascular decompression and partial trigeminal rhizotomy.
Adapted from Taha JM, Tew JM. Comparison of surgical treatments for trigeminal neuralgia reevaluation of radiofrequency rhizotomy. Neurosurgery 1996;38:865–871.
RF ablation of the trigeminal ganglion has been in use for decades, and there are large retrospective series conducted in a similar fashion to guide our understanding of the usefulness and complications associated with this approach. In current times, the use has declined and is now limited to those patients who have failed pharmacologic therapy and are not deemed medically fit or decline to undergo microvascular decompression.17 The initial success rates for all surgical interventions range from 92% to 98% (Table 32-2). The rate of pain recurrence among percutaneous techniques is lowest with RF rhizotomy (20% in 9 years) when compared with glycerol rhizotomy (54% in 4 years) and balloon compression (21% in 2 years).17 The most common complications and adverse effects (some of which are an expected part of the treatment) include facial numbness (98%), dysesthesia (24%), anesthesia dolorosa (1.5%), corneal anesthesia (7%), keratitis (1%), and trigeminal motor dysfunction (24%) (Table 32-2). A recent review details the range of incidence of complications from the published literature (Table 32-3).19
TABLE 32.3 Complications of RF Rhizotomy for Trigeminal Neuralgia
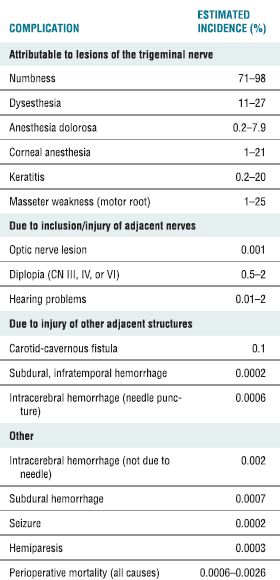
Adapted from Lord SM, Bogduk N. Radiofrequency procedures in chronic pain. Best Pract Res Clin Anesthesiol 2002;16:597–617.
Mechanism of Injury
The mechanism of injury during RF rhizotomy for trigeminal neuralgia may be related to injury caused during placement of the cannula or injury that results from the thermal destruction during RF treatment. The RF cannula is inserted through the skin just adjacent to the lateral margin of the mouth and cephalad via the foramen ovale in the base of the skull until the active tip lies adjacent to or within the trigeminal ganglion. Placement of the cannula generally requires brief intervals of deep sedation. En route to the trigeminal ganglion, the cannula courses medial to the body of the mandible just beneath the oral mucosa, and there is significant risk of piercing the oral mucosa and dragging bacteria into the cranium. Meningitis is a rare complication, and the exact portal of bacterial entry is usually uncertain.20,21 Within the skull, the trigeminal ganglion lies lateral to the posterior clinoid process of the sella turcica, just lateral to the internal carotid artery, anterior to the pons, and medial to the inferomedial aspect of the temporal lobe of the brain (Fig. 32-2). Excessive advancement of the needle risks injury to these structures. Indeed, case reports of brain stem injury have appeared.22 Excessive anterior angulation of the advancing cannula can lead it through the pterygopalatine fossa up through the inferior orbital fissure and into the optic canal, where optic nerve injury and monocular blindness have occurred.23 In addition, toward its posterior aspect, the trigeminal ganglion is enveloped closely within dural reflections, and even proper placement of the cannula often results in the appearance of cerebrospinal fluid (CSF) flowing freely from the cannula. This rarely creates any longterm sequelae, but may lead to reports of CSF rhinorrhea,24 believed to be retrograde flow of CSF through the foramen ovale and into the auditory (Eustachian) tube through an opening in the canal created during needle placement.
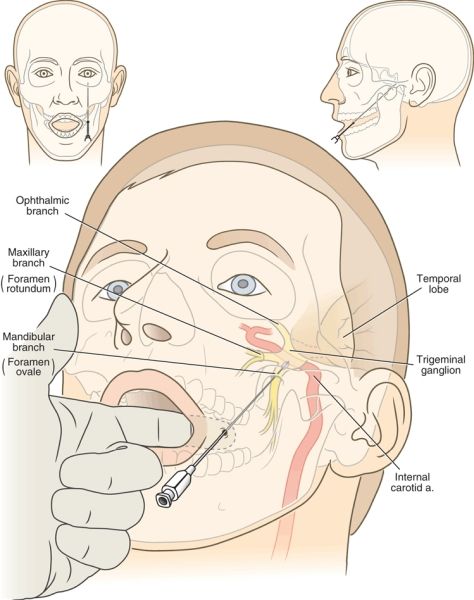
FIGURE 32-2. Mechanism of complications that arise during RF treatment for trigeminal neuralgia. The RF cannula is inserted just lateral to the lateral margin of the lips and advanced medial to the mandible and lateral to the oral mucosa. The index finger of the operator is placed in the patient’s mouth to ensure that the tip of the cannula does not penetrate the oral mucosa en route to the foramen ovale. Note the close proximity of the carotid artery, the temporal lobe, and the brain stem to the final position of the cannula (insets). The proper trajectory of the needle and final needle position are shown in the frontal and axial planes.
Neural injury during RF ablation of the trigeminal ganglion is universal and is indeed the goal of this treatment modality. Direct destruction of a small area of neurons within the distal portion of the ganglion or the nerve rootlets is believed to be the mechanism that interrupts the episodic pain. To target the RF treatment area, the cannula is advanced until a low-voltage high-frequency current produces a paresthesia within the area where the patient typically experiences pain. Thereafter, heat lesioning is begun for 60-second intervals at 60°C under brief periods of general anesthesia. Between treatments, the patient is allowed to recover enough to allow sensory testing within the cutaneous distribution where the pain is localized. Repeated lesions are created at gradually increasing temperatures (typically 2°C increments) to increase the degree of neural destruction until the patient reports the appearance of slight hypesthesia in the affected region. The most common adverse effects of this approach are related to the size and location of the thermal lesion (Tables 32-2 and 32-3). Judging the extent of the sensory change following each lesion can be quite difficult and is often clouded by effects of the anesthetic. If the extent of the lesion is excessive, the area of sensory loss can extend to the ophthalmic division of the trigeminal nerve (VI) and can result in loss of the corneal reflex and corneal anesthesia, and keratitis may ensue. The majority of patients (98%)17 will report some detectable degree of sensory loss within the region treated, and this loss of sensation can be bothersome to some. Others have reported dense sensory anesthesia and ongoing neuropathic pain in the region of treatment (anesthesia dolorosa). Treatment of the mandibular division of the trigeminal nerve (V3) involves motor fibers to the mas-seter muscle and weakness during mastication is common. Finally, direct heating of the trigeminal rootlets can lead to dramatic rises in blood pressure and intracranial bleeding.25 In a recent large review examining 6,205 patients treated with RF rhizotomy for trigeminal neuralgia, this complication has not been reported.17 Perhaps due to the advent of more rapidly acting sedative hypnotics and meticulous intra-operative monitoring, such dangerous swings in blood pressure have become less common.
Diagnosis
Diagnosis of adverse effects and complications associated with RF treatment for trigeminal neuralgia is usually self-evident. Masticatory weakness, variable degrees of hypesthesia, and anesthesia dolorosa occur with sufficient frequency that they should be expected and patients should be counseled regarding each as a part of the informed consent process prior to treatment. CSF rhinorrhea is typically painless and self-limited. New onset of a focal neurologic deficit or headache after treatment warrants immediate imaging of the brain using MRI to rule out hematoma formation with reversible compression of neural elements. Although direct neural injury is by far the more common cause leading to new focal deficits, a reversible cause should be ruled out. Indeed, this technique has been largely performed by neurosurgeons. Ongoing and close collaboration with a neurosurgeon is warranted for anesthesiologists and others who perform the technique independently, and immediate neurosurgical consultation should be readily available whenever the technique is used. For those patients who develop corneal anesthesia following the procedure, consultation with an ophthalmologist should be arranged to guide treatment aimed at preventing keratitis.
Treatment
Treatment of the most common adverse effects associated with RF treatment for trigeminal neuralgia is symptomatic. Most problems are self-limited. Like other forms of neuropathic pain stemming from neural injury, anesthesia dolorosa is difficult to treat. The use of antiepileptic and antidepressant drugs is the cornerstone of management for this problem. In the immediate time interval following RF treatment, the pain can be extreme, and use of opioid analgesics (often in high doses) is the only available means of temporizing. Onset of a new focal neurologic deficit may herald significant direct neural trauma. Therapy is guided by diagnostic imaging. In the event that significant intracranial bleeding occurs, immediate surgical decompression may be warranted.
Prevention
Central to successful application of RF treatment for trigeminal neuralgia is the use of meticulous image-guided placement of the RF cannula and creation of the smallest anatomically correct lesion that will produce mild hypesthesia in the region affected. Correct needle placement begins with advancing the needle over the medial aspect of the mandible beneath the oral mucosa. Penetration of the oral mucosa and further advancing the needle risks seeding the intracranial vault with oral bacteria. This can be avoided by placing a gloved hand in the patient’s mouth and palpating the needle beneath the oral mucosa as it is advanced. Once the needle has been advanced beyond the posterior extent of the mandible toward the base of the skull, there is no further risk of penetrating the oral mucosa. Trauma to extracranial vascular structures near the skull base, including the carotid artery as it enters the carotid canal, is minimized by use of fluoroscopic guidance. The foramen ovale should be identified near the base of the lateral pterygoid plate, and excessive deviation from this location should be avoided. Once the needle has entered the foramen ovale, fluoroscopic images in the AP and lateral planes should be used to avoid excessive cranial advancement. The needle tip should not be inserted beyond a line drawn between the superior aspects of the anterior and posterior clinoid processes in a lateral radiograph to avoid direct trauma to the temporal lobe and brain stem (Fig. 32-2). Sensory stimulation should then be carried out just after traversing the foramen. Repeated sensory stimulation after each small (2- to 3-mm) advance of the needle will ensure that the needle reaches the proper position without excessive advancement within the cranium.
For those patients with involvement of the ophthalmic division of the trigeminal nerve (V1), many experts question the usefulness of RF treatment.17 Because thermal injury to the ganglion to the point of sensory loss is required for successful treatment, some degree of corneal anesthesia is to be expected if the lesion is targeted to this region of the ganglion. Nonetheless, when other treatment options are not available even those with involvement of V1 have been successfully treated, albeit at a heightened risk of corneal anesthesia and subsequent keratitis.
 RADIOFREQUENCY TREATMENT FOR FACET-RELATED PAIN
RADIOFREQUENCY TREATMENT FOR FACET-RELATED PAIN
Scope
Neck and low back pain are ubiquitous and have many causes. Each element of the spine can give rise to distinct pain syndromes. Facet-related pain has been recognized as a common cause of axial neck and low back pain (i.e., pain that is primarily along the spinal axis as opposed to radicular pain that extends into the extremities and suggests neural compression).26 The diagnosis of facet-related pain is made on the basis of symptoms. There are no definitive diagnostic studies, and, thus, the diagnosis is imprecise at best. Patients with facet-related pain typically report deep aching pain that predominantly overlies the spinal axis. The pain may be unilateral or bilateral. The most common causes of facet-related pain appear to be degenerative changes within the facet joints, most often osteoarthritis (“spondylosis”). There may be some history of trauma, particularly in facet-related neck pain. The most common injuries are sudden twisting or flexion-extension injuries (e.g., whiplash). Symptoms are usually exacerbated by extension. There are well-documented patterns of referred pain arising from various facet joints that help with deciding where treatment should be focused.2729 Imaging studies (CT, MRI) may be completely normal, even though facet arthropathy of varying degrees is quite common. Most clinicians rely on diagnostic injections to guide patient selection for RF facet denervation. Intra-articular facet injections or blocks of the medial branch nerves to the facet using local anesthetic should produce transient relief of the symptoms before proceeding with RF treatment.
Despite widespread use of RF thermoablation for facet-related pain, there is limited data regarding the safety of this technique and its associated complications. No formal safety assessment has been performed.30 In the absence of such formal assessment, knowledge must be gleaned from review of published studies. Attempts to evaluate incidence and severity of complications are frustrated by variability in the published literature. Authors have differed widely regarding the detail with which complications have been reported, and some authors have neither reported complications nor remarked on their absence. Nonetheless, numerous reports have confirmed the safety of RF facet denervation. Several large series of patients treated with lumbar RF denervation have been reported without major complications.31 Notably, there have been few reports of major neurologic injury resulting from this technique, and it is rare to see anything more than transient exacerbation of symptoms following RF facet denervation. The primary limitations of this technique are the rate of failure and return of pain after treatment.
It is clear that less than half of reported patients that undergo diagnostic blocks proceed to neurolysis. Of those undergoing lumbar facet denervation, about half of patients obtain good to excellent pain relief. In a summary report of numerous uncontrolled studies, the proportion of patients achieving >50% pain relief varied from 17% to 82%, with a mean success rate of 48%. However, the proportion of patients obtaining complete relief was unstated, and the duration of follow-up was often less than a year.32 In contrast, more recent studies have incorporated rigorous patient selection criteria and sham controls. Among the best designed and conducted of trials was a recent prospective, randomized, placebo- (sham) controlled study of patients with cervical pain (whiplash) that demonstrated 50% pain reduction for a median of 9 months after facet denervation versus 8 days in the sham-treated group.33 A recent systematic review concluded that percutaneous RF neurolysis of the sensory nerves to the facet joints is a safe and modestly effective treatment for facet-related pain.34 While dozens of observational or retrospective studies have reported varying clinical results with the use of pulsed RF treatment, only one, small well-controlled randomized trial has appeared in recent years. Van Zundert et al.35 and his colleagues randomized 23 patients with chronic cervical radicular pain to receive either pulsed RF treatment for the dorsal root ganglion (DRG) at the painful level or sham treatment and showed superior pain reduction lasting to 6-month follow-up in those receiving active treatment.35 This technique is in need of further study to validate its usefulness.
A recent review details the adverse effects and complications associated with cervical RF neurotomy (Table 32-4).17 In a series of 28 patients who received cervical RF treatment, McDonald et al.36 reported no complications. In a comment on this article, Burchiel37 asserted the importance of warning patients about the possibility of both cutaneous dysesthesia and postoperative pain and numbness that could last between 2 and 34 months,37 both of which are common sequelae of cervical RF neurotomy that are often considered expected outcomes rather than complications. The most common complications associated with cervical medial branch neurotomy include postoperative pain (97%); ataxia, unsteadiness, and spatial disorientation (23%); and vasova-gal syncope (2%) (Table 32-4). Lord et al.38 found that brief postoperative pain was the only side effect of lower cervical procedures, but ataxia occurred when the third occipital nerve was treated. Because the third occipital nerve carries a large proportion of fibers involved in cutaneous innervation, numbness routinely accompanies lesioning of this nerve. In patients whose treatment was successful, this numb patch regressed over 1 to 3 weeks and was replaced by dysesthesia and pruritis, followed by the return of normal cutaneous sensation and pain. Unsuccessful treatment was characterized by the loss of numbness and return of pain within 1 week.
TABLE 32.4 Observed Complications of Cervical Medial Branch Neurotomy
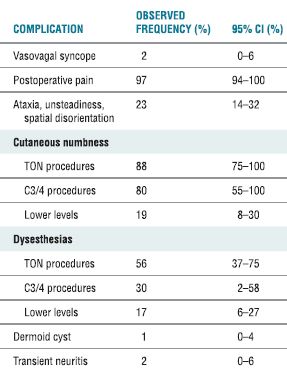
CI, confidence interval; TON, third occipital nerve.
Modified from Lord SM, Bogduk N. Radiofrequency procedures in chronic pain. Best Pract Res Clin Anesthesiol 2002;16:597–617.



