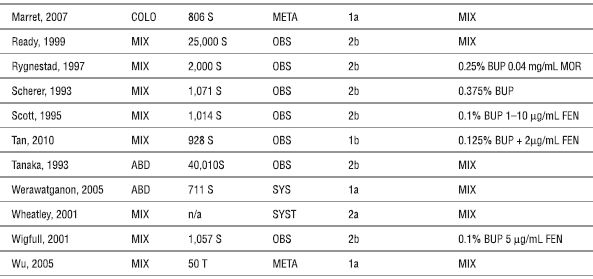
ABD (abdominal), BUP (bupivacaine), CAN (cancer), COLO (colorectal), FEN (fentanyl), META (meta-analysis), MIX (mixed), n/a (not available), MOR (morphine), OBS (observational study), S (subjects), SUF (sufentanil), SYST (systematic review), T (trials), and THOR (thoracic).
aLevel of evidence based on recommendation from the Oxford Centre for Evidence-based Medicine Levels of Evidence (2001) (http://www.cebm.net/index.aspx?o=1025; accessed December 22, 2010).
Block BM, Liu SS, Rowlingson AJ, et al. Efficacy of postoperative epidural analgesia: a meta-analysis. JAMA 2003;290:2455–2463.
Brodner G, Mertes N, Buerkle H, et al. Acute pain management: analysis, implications and consequences after prospective experience with 6349 surgical patients. Eur J Anaesthesiol 2000;17:566–575.
Broekema AA, Gielen MJ, Hennis PJ. Postoperative analgesia with continuous epidural sufentanil and bupivacaine: a prospective study in 614 patients. Anesth Analg 1996;82:754–759.
Burstal R, Wegener F, Hayes C, et al. Epidural analgesia: prospective audit of 1062 patients. Anaesth Intensive Care 1998;26:165–172.
Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth 2004;93:212–223.
de Leon-Casasola OA, Parker B, Lema MJ, et al. Postoperative epidural bupivacaine-morphine therapy. Experience with 4,227 surgical cancer patients. Anesthesiology 1994;81:368–375.
Dolin SJ, Cashman JN, Bland JM. Effectiveness of acute postoperative pain management: I. Evidence from published data. Br J Anaesth 2002;89:409–423.
Flisberg P, Rudin A, Linner R, et al. Pain relief and safety after major surgery. A prospective study of epidural and intravenous analgesia in 2696 patients. Acta Anaesthesiol Scand 2003;47:457–465.
Liu SS, Bieltz M, Wukovits B et al. Prospective survey of patient-controlled epidural analgesia with bupivacaine and hydromorphone in 3736 postoperative orthopedic patients. Reg Anesth Pain Med 2010;35:351–354.
Liu SS, Allen HW, Olsson GL. Patient-controlled epidural analgesia with bupivacaine and fentanyl on hospital wards: prospective experience with 1,030 surgical patients. Anesthesiology 1998;88:688–695.
Lubenow TR, Faber LP, McCarthy RJ, et al. Postthoracotomy pain management using continuous epidural analgesia in 1,324 patients. Ann Thorac Surg 1994;58:924–929.
Marret E, Remy C, Bonnet F. Meta-analysis of epidural analgesia versus parenteral opioid analgesia after colorectal surgery. Br J Anaesth 2007;94:665–673.
Ready LB. Acute pain: lessons learned from 25,000 patients. Reg Anesth Pain Med 1999;24:499–505.
Rygnestad T, Borchgrevink PC, Eide E. Postoperative epidural infusion of morphine and bupivacaine is safe on surgical wards. Organisation of the treatment, effects and side-effects in 2000 consecutive patients. Acta Anaesthesiol Scand 1997;41:868–876.
Scherer R, Schmutzler M, Giebler R, et al. Complications related to thoracic epidural analgesia: a prospective study in 1071 surgical patients. Acta Anaesthesiol Scand 1993;37:370–374.
Scott DA, Beilby DS, McClymont C. Postoperative analgesia using epidural infusions of fentanyl with bupivacaine. A prospective analysis of 1,014 patients. Anesthesiology 1995;83:727–737.
Tan T, Wilson D, Walsh A, et al. Audit of a ward-based patient-controlled epidural analgesia service in Ireland. Ir J Med Sci 2011;180:417–421
Tanaka K, Watanabe R, Harada T, et al. Extensive application of epidural anesthesia and analgesia in a university hospital: incidence of complications related to technique. Reg Anesth 1993;18:34–38.
Werawatganon T, Charuluxanun S. Patient controlled intravenous opioid analgesia versus continuous epidural analgesia for pain after intra-abdominal surgery. Cochrane Database Syst Rev 2005;(1):CD004088.
Wheatley RG, Schug SA, Watson D. Safety and efficacy of postoperative epidural analgesia. Br J Anaesth 2001;87:47–61.
Wigfull J, Welchew E. Survey of 1057 patients receiving postoperative patient-controlled epidural analgesia. Anaesthesia 2001;56:70–75.
Wu CL, Cohen SR, Richman JM, et al. Efficacy of postoperative patient-controlled and continuous infusion epidural analgesia versus intravenous patient-controlled analgesia with opioids: a meta-analysis. Anesthesiology 2005;103:1079–1088.
Despite the potential benefits of continuous epidural analgesia for postoperative pain management, there are risks and complications associated with the use of this technique. The majority of these complications (e.g., nausea, vomiting, itching, urinary retention, motor block, catheter failure) are not life threatening but may be bothersome for the patient, decrease patient satisfaction and quality of recovery, or even delay postoperative convalescence. Other complications are much less common (e.g., intravascular or intrathecal migration), and some can result in permanent and devastating injuries (e.g., epidural hematoma or abscess).
In general, complications from continuous epidural analgesia can be categorized as medication related or catheter related. Medication-related complications include nausea, vomiting, pruritus, motor block, hypotension, and respiratory depression. Catheter-related complications include catheter failure/dislodgement, intrathecal or intravascular migration, and epidural hematoma or abscess. Many of the rare but devastating complications that may be associated with continuous epidural analgesia are described in greater detail in other chapters (Chapter 4, Bleeding Complications; Chapter 5, Infectious Complications; Chapter 7, Local Anesthetic Systemic Toxicity; and Chapter 11, Local Anesthetic Neurotoxicity and Cauda Equina). In this chapter, we focus on some of the more common medication- and catheter-related side effects and complications associated with continuous epidural analgesia.
 MEDICATION-RELATED COMPLICATIONS
MEDICATION-RELATED COMPLICATIONS
Although many agents may be used for postoperative epidural analgesia, the two most common agents administered for continuous epidural analgesia are opioids and local anesthetics. As would be expected, there are differences in the incidences of side effects between epidurally administered opioids and local anesthetic. Opioids and local anesthetic are typically administered in tandem, as this combination may provide at least additive if not synergistic analgesia while minimizing the incidence of complications from each agent. Although the optimal epidural local anesthetic-opioid combinations are unknown, bupivacaine, ropivacaine, or levobupivacaine (≤0.125% bupivacaine or levobupivacaine, or ≤0.2% ropivacaine) are the local anesthetics typically chosen due to their differential and preferential clinical sensory-motor blockade10–12. A lipophilic opioid such as fentanyl (2–5 μg/mL) or sufentanil (0.5–1 μg/mL) is commonly used to allow for rapid titration of analgesia, especially if patient-controlled analgesia is chosen,13–15 although a hydrophilic opioid (morphine 0.05–0.1 mg/mL or hydromorphone 0.01–0.05 mg/mL) added to a local anesthetic will also provide effective postoperative analgesia.13,15 However, both opioids and local anesthetics may be used as the sole agent to provide continuous epidural analgesia. It should be noted that there may be a wide range of incidences quoted for each complication, which may reflect the variability in how each complication is defined (and thus recorded) in different studies.
Complications Associated with Epidural Administration of Opioids
When opioids are used as a solo agent for continuous postoperative epidural infusions, they offer several advantages over continuous local anesthetic epidural infusions in that they do not generally cause motor block or hypotension.14 Epidural opioid infusions, however, may be associated with nausea, vomiting, pruritus, and respiratory depression (Fig. 20-1). These side effects are similar to those seen with systemic administration of opioids. Although lipophilic agents such as fentanyl and sufentanil may be used for continuous epidural infusions, the analgesic site of action for continuous epidural infusions of lipophilic opioids appears to be systemic rather than spinal,16–18 as randomized controlled trials indicate no differences in plasma concentrations, side effects, or pain scores between those randomized to receive either intravenous or epidural infusion of fentanyl.16,17 On the other hand, there may be more utility in administering a continuous epidural infusion of a hydrophilic opioid such as morphine or hydromorphone, where the analgesic site of action is primarily spinal,15 which may be particularly useful in situations where epidural catheter insertion is not congruent with the site of surgery or when local anesthetic-related side effects (e.g., hypotension, motor block) interfere with the efficacy of postoperative epidural analgesia. Although a single dose or intermittent boluses of a hydrophilic opioid may be administered epidurally for effective postoperative analgesia, use of a continuous infusion may result in superior analgesia with fewer side effects15,19 and may provide superior analgesia compared to traditional “as-needed” administration of systemic opioids.20,21
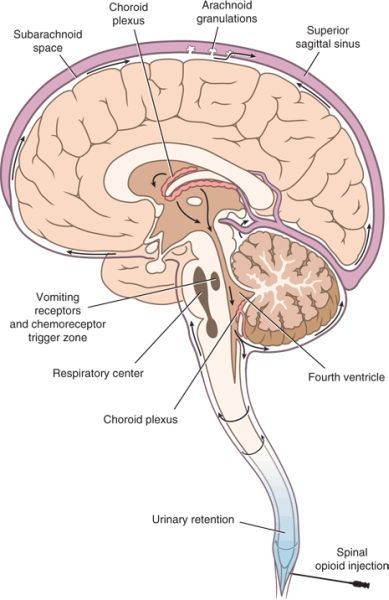
FIGURE 20-1. Anatomic sites of opioid action following neuraxial administration. Opioid administered along the neuraxis reaches the CSF, either directly during intrathecal administration or after diffusion from the epidural space through the dura and into the CSF. Within the CSF, the opioid circulates to more rostral centers within the CNS. Binding to specific receptors produces predictable opioid-related effects. Analgesia results from binding to specific receptors within the dorsal horn of the spinal cord and higher centers within the brain stem, notably the periaqueductal gray matter. Nausea and vomiting result from binding to receptors within the floor of the fourth ventricle, termed the chemoreceptor trigger zone. Binding to specific receptors within the vasomotor and respiratory centers located in the medulla oblongata produces the characteristic increase in vagal tone and respiratory depression associated with opioids.
Nausea and Vomiting
Scope of the Problem. Postoperative nausea and vomiting (PONV) associated with continuous epidural analgesia is relatively common, with an overall incidence for continuous epidural analgesia ranging from 3% to 60% (Table 20-2 and Box 20-1). A meta-analysis of randomized trials examining continuous epidural analgesia indicated that continuous epidural analgesia solutions consisting of a local anesthetic-based regimen (with or without opioid) appeared to generally have a lower incidence of PONV compared with an opioid-only regimen (i.e., up to 42% PONV with a local anesthetic-based solution vs. up to 60% PONV with opioids alone).1 The selection of opioid appears to influence the incidence of PONV associated with continuous epidural analgesia, as the use of fentanyl (either alone or in combination with a local anesthetic) is associated with a lower incidence of PONV than continuous epidural infusions containing morphine.20–24 In addition, the cumulative incidence of PONV may be higher in those receiving continuous infusions of opioids rather than a single-shot dose (45%–80% vs. 20%–50%).22,23,25,26
BOX 20-1 Incidence of Nausea and Vomiting During Epidural Analgesia
 Infusions of opioid alone > combined opioid + local anesthetic infusions
Infusions of opioid alone > combined opioid + local anesthetic infusions
 Morphine > fentanyl
Morphine > fentanyl
 Continuous infusion > single-shot epidural injections (cumulative incidence)
Continuous infusion > single-shot epidural injections (cumulative incidence)
TABLE 20.2 Overall Incidence of Side Effects and Complications of Continuous Postoperative Epidural Analgesiaa
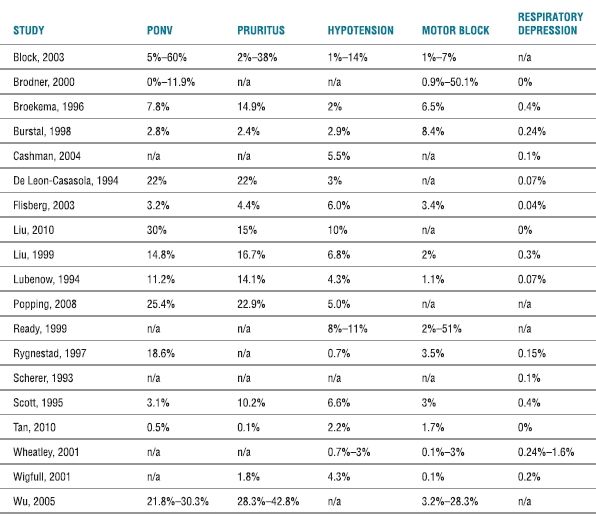
aComplications as defined by the articles referenced. A range is given for systematic reviews. For multiple entries from individual studies (e.g., measured incidence of PONV for each POD), the highest incidence recorded is provided.
N (nausea); V (vomiting).
Pathophysiology. PONV from neuraxial opioids is related to the activation of the chemoreceptor trigger zone and area postrema in the medulla resulting from the cephalad migration of opioid within the cerebrospinal fluid.27 Based on both clinical and experimental data, the incidence of PONV from neuraxial opioids appears to be dose dependent.28–31
Prevention and Treatment. Although a variety of agents (including naloxone, droperidol, metaclopramide, dexamethasone, and transdermal scopolamine) may be used to treat PONV, the majority of trials have examined these agents for the prevention and treatment of PONV associated with primarily single-shot neuraxial opioids, not continuous epidural infusions of opioids25,26,32,33 (Box 20-2). One of the few studies to examine an intervention for PONV after continuous epidural analgesia with morphine was performed by Nakata et al., in which 120 patients undergoing thoracic or abdominal surgery received an intraoperative epidural injection of 2 mg morphine followed by a postoperative continuous epidural infusion of 4 mg/d of morphine.26 Patients were randomized to receive saline, a single intraoperative dose of intravenous droperidol (2.5 mg), a postoperative continuous epidural droperidol infusion (2.5 mg/d), or a single intraoperative dose of intravenous droperidol (2.5 mg) followed by a postoperative continuous epidural infusion of droperidol (2.5 mg/d). Both intravenous and epidural droperidol significantly reduced the frequency and severity of PONV induced by continuous epidural morphine.26
BOX 20-2 Treatments with Proven Benefit for Nausea and Vomiting Associated with Epidural Analgesia
 Intravenous dexamethasone
Intravenous dexamethasone
 Intravenous droperidol
Intravenous droperidol
 Transdermal scopolamine
Transdermal scopolamine
 Equivocal: ondansetron/tropisetron (serotonin receptor antagonists)
Equivocal: ondansetron/tropisetron (serotonin receptor antagonists)
Other randomized controlled trials have examined various interventions for the prevention and treatment of PONV resulting from a single-shot administration of opioid (e.g., spinal morphine for postoperative analgesia after cesarean delivery). All of the agents (serotonin receptor antagonists, dexamethasone, droperidol, metoclopramide, scopolamine transdermal) examined appear to be relatively efficacious in preventing or treating PONV associated with neuraxial opioids, although the data supporting the efficacy of each agent are somewhat equivocal. Dexamethasone (5 mg intravenously) appears to have the most consistent benefit for PONV associated with single-shot neuraxial opioids, with at least six randomized controlled trials supporting its benefit in the prevention of PONV associated with neuraxial opioids.25,34–38 The patients in these studies were females undergoing a variety of gynecologic and obstetric procedures. However, two other studies39,40 did not demonstrate a benefit of dexamethasone for PONV associated with neuraxial opioids. Droperidol and transdermal scopolamine also appear to be efficacious in reducing PONV associated with neuraxial opioids,35,41–43 although these agents may not be commonly used in part due to some safety concerns (droperidol) or side effects (transdermal scopolamine).44,45 Finally, the efficacy of serotonin receptor antagonists (e.g., ondansteron, tropisetron) on PONV associated with neuraxial opioids is equivocal, with some randomized controlled trials demonstrating a beneficial effect in reducing the incidence of PONV39,46 while other studies showing no benefit.36,47
Pruritus
Scope of the Problem. Pruritus is a relatively common side effect from continuous epidural analgesia, especially for those analgesic regimens containing opioids1 (Box 20-3). The overall incidence of pruritus for continuous epidural analgesia ranges from 2% to 38% (Table 20-2), although a meta-analysis indicated that continuous epidural analgesic solutions consisting of an opioid alone (incidence of ~7%–38%) will result in a higher incidence of pruritus than an epidural regimen containing a local anesthetic and opioid (incidence reported at ~2%).1 Using fentanyl instead of morphine in the epidural analgesic solution appears to be generally associated with a lower incidence of pruritus.22,24,48 The higher incidence of pruritus with continuous infusions of epidural opioids (which may be as high as 60% to 100% compared with a lower incidence of ~15%–18% with epidural local anesthetic administration or systemic opioids48–50) is similar to that seen with single-shot intrathecal opioid (typically morphine).
BOX 20-3 Incidence of Pruritus Associated with Epidural Analgesia
 Opioid alone > opioid + local anesthetic
Opioid alone > opioid + local anesthetic
 Morphine > fentanyl
Morphine > fentanyl
 Continuous infusion ≅ single shot
Continuous infusion ≅ single shot
Pathophysiology. The etiology of neuraxial opioid-induced pruritus is unclear, although there are several theories. One postulated mechanism includes the presence of an “itch” center, similar to the emesis center for PONV.51 Experimental studies suggest that this itch center may be located in the medullary dorsal horn or lower medulla that includes the trigeminal nucleus.51,52 It does appear that the μ-opioid receptor may in part mediate neuraxial opioid-induced pruritus possibly via opioid antagonism of central inhibitory neurotransmitters (e.g., gamma-aminobutyric acid and glycine).51,53 In addition, other receptors (especially the serotonin receptors) may play a role in neuraxial opioid-induced pruritus.51 Peripheral histamine release does not appear to be an important etiology of neuraxial induced opioid pruritus. Clinical and experimental data suggest that there is a dose-dependent relationship between the incidence of pruritus and the dose of neuraxial opioid.54–57 However, a quantitative systematic review seems to indicate otherwise.50
Prevention and Treatment. A variety of agents have been used for the prevention and treatment of neuraxial opioid-induced pruritus, although the vast majority of studies have evaluated these agents primarily after a single-shot intrathecal dose of opioid (typically morphine) and not with continuous epidural infusions of opioid (Box 20-4). These agents include opioid antagonists, propofol, droperidol, and serotonin receptor antagonists. A systematic review indicated that both pure opioid antagonists (e.g., naloxone) and opioid agonist-antagonists (e.g., nalbuphine) were efficacious in the prevention of neuraxial opioid-induced pruritus. However, the higher doses of these agents (e.g., >2 μg/kg/h of naloxone)—which were more efficacious in preventing neuraxial opioid-induced pruritus—also decreased the quality of analgesia.50 A randomized trial comparing nalbuphine (40 mg intravenous dose) to placebo indicated that nalbuphine was efficacious in preventing pruritus but was associated with increased drowsiness.51 An interesting option for the prevention and treatment of neuraxial opioid-induced pruritus is propofol in subhypnotic doses. Randomized data in nonobstetric patients indicate that propofol is efficacious in preventing (10 mg bolus followed by 30 mg over 24 hours) and treating (10-mg bolus) neuraxial opioid-induced pruritus.58,59 However, randomized controlled trials in obstetric patients do not confirm these benefits of subhypnotic doses of propofol.60,61 Finally, because of the possible involvement of the serotonin receptor in mediating neuraxial opioid-induced pruritus, there have been several randomized controlled trials examining the efficacy of serotonin receptor antagonists (e.g., ondansetron) for the prevention or treatment of the incidence or severity of pruritus.46,62–68 Two systematic reviews provide somewhat conflicting results, with an earlier systematic review suggesting that serotonin receptor antagonists significantly reduced the odds of pruritus (number needed to treat = 6) and intensity/treatment requests for pruritus69 while a more recent meta-analysis indicated that serotonin receptor antagonists were ineffective in reducing the incidence of pruritus in patients undergoing Cesarean section, although the severity and need for treatment were improved with serotonin receptor antagonists.70
BOX 20-4 Treatments with Proven Benefit for Pruritus Associated with Epidural Analgesia
 Naloxone
Naloxone
 Nalbuphine
Nalbuphine
 Low-dose propofol (nonobstetric patients)
Low-dose propofol (nonobstetric patients)
Respiratory Depression
Scope of the Problem. A relatively uncommon but potentially life-threatening complication of single-shot or continuous epidural infusion of neuraxial opioids is respiratory depression (Box 20-5). The incidence of neuraxial opioid-related respiratory depression may in part depend on the wide variety of definitions for respiratory depression used in individual trials (e.g., need for naloxone, ventilatory frequency, oxygen saturation below a predetermined level, or PaCO2 above a predetermined level).71 However, large-scale trials indicate that the overall incidence (typically based on the use of naloxone) appears to be <1% (Table 20-2). The incidence of respiratory depression associated with neuraxial administration of opioids is dose dependent and typically ranges from 0.04% to 1.6% (Table 20-2). A systematic review of 165 trials (which included a total of 50,642 patients with epidural analgesia) noted a mean incidence of respiratory depression necessitating naloxone use of only 0.1% (95% confidence interval [CI] of 0.1%–0.2%).71 When used appropriately, neuraxial opioids (either as a single-shot or continuous infusion) do not appear to have a higher incidence of respiratory depression than that seen with systemic administration of opioids.71,72 In fact, in one systematic review, the mean incidence of respiratory depression (necessitating naloxone use) for epidural analgesia (0.1% with 95% CI of 0.1%–0.2%) was significantly lower than that from intravenous patient-controlled analgesia with opioids (1.9% with 95% CI of 1.9%–2.0%).71 Although the necessity of intensive care–like monitoring for patients who receive neuraxial opioids is controversial, many large-scale trials have demonstrated the relative safety (incidence of respiratory depression <0.9%) of this technique on regular surgical wards.73–76
BOX 20-5 Incidence of Respiratory Depression Associated with Epidural Analgesia
 Respiratory depression requiring naloxone administration occurs in <1% of patients receiving epidural analgesia.
Respiratory depression requiring naloxone administration occurs in <1% of patients receiving epidural analgesia.
 The incidence of respiratory depression associated with neuraxial administration of opioids appears to be similar to that following systemic administration of opioids.
The incidence of respiratory depression associated with neuraxial administration of opioids appears to be similar to that following systemic administration of opioids.
Pathophysiology. The mechanism of neuraxial opioid-related respiratory depression is related to the interaction of the neuraxial opioid (whether it be systematically via absorption into the vasculature [i.e., early respiratory depression] or spinally via cephalad spread through the cerebrospinal fluid [i.e., delayed respiratory depression]) with the opioid receptors in ventral respiratory group in the brain stem, although the exact sites for opioid-induced effects on respiration have not yet been elucidated.77,78 Although neuraxial administration of lipophilic opioids (e.g., fentanyl, sufentanil) may be associated with early respiratory depression due to the relatively greater systemic absorption, lipophilic opioids are generally considered to have a lower incidence of delayed respiratory depression than hydrophilic opioids (e.g., morphine, hydromorphone).79–81 Delayed respiratory depression with neuraxial administration of hydrophilic opioids is generally related to the cephalad spread of opioid in the cerebrospinal fluid, which typically occurs within 12 hours following injection.82
Risk Factors. Although the risk factors for neuraxial opioid-related respiratory depression have not been clearly elucidated, some identified risk factors include increasing dose, increasing age, concomitant use of systemic opioids or sedatives, and possibly prolonged or extensive surgery, presence of comorbidities, and thoracic surgery82 (Box 20-6). In addition, more recent data suggest that the use of extended-release epidural morphine may be associated with significantly higher odds of respiratory depression compared to intravenous patient-controlled analgesia with opioids.83
BOX 20-6 Risk Factors for Neuraxial Opioid-induced Respiratory Depression
 Increasing age
Increasing age
 Increasing dose
Increasing dose
 Concomitant use of systemic opioids or sedatives
Concomitant use of systemic opioids or sedatives
 Presence of comorbidities (e.g., chronic pulmonary disease, obstructive sleep apnea)
Presence of comorbidities (e.g., chronic pulmonary disease, obstructive sleep apnea)
 Thoracic surgery
Thoracic surgery

Full access? Get Clinical Tree


