Coagulopathy in the critically ill covers a range of abnormal states of coagulation. It is defined as the blood’s inability to clot normally.
I. COAGULOPATHIES AND HEMOSTATIC ABNORMALITIES IN THE CRITICALLY ILL
A. Disseminated Intravascular Coagulation (DIC) is defined by the International Society on Thrombosis and Hemostasis as an “acquired syndrome characterized by the intravascular activation of coagulation with loss of localization arising from different causes.” There are many potential causes of DIC (Table 25.1).
1. Pathophysiology of DIC is based on the following main mechanisms (Fig. 25.1). First, bacterial exo- and endotoxins (LPS) as well as inflammatory cytokines induce tissue factor-expression on circulation monocytes and endothelial cells. This results in a loss of localization of thrombin generation to the site of endothelial injury. Tissue factor-expression on monocytes is further enhanced by the surfaces of extracorporeal assist devices such as dialysis, ECMO, and ventricular assist devices (VADs). However, tissue factor-expression on monocytes cannot be detected by standard plasmatic coagulation testing but by whole-blood viscoelastic testing (ROTEM/TEG). Second, the early phase of DIC is characterized by hypercoagulability (increased clot firmness due to acute phase reaction with high fibrinogen levels and high clot firmness in viscoelastic testing), consumption of physiologic coagulation inhibitors (antithrombin and protein C), platelet dysfunction, and inhibition of fibrinolysis (up-regulation of plasmin activator inhibitor-1 [PAI-1]). This results in thrombosis of the microcirculation and multiple organ failure. Finally, when coagulation factors and fibrinolytic inhibitors are consumed, hypocoagulation and secondary fibrinolysis can result in severe bleeding. Early detection of pathologic thromboelastometric results and platelet dysfunction detected by whole-blood impedance aggregometry on admission at the ICU are associated with worse outcomes.
| Causes of Disseminated Intravascular Coagulation (DIC) | |
Acute | Chronic |
Sepsis | Malignancy (hematologic or solid organ) |
Shock | Liver disease |
Trauma | Vascular abnormalities |
Head injury | Aortic aneurysm |
Crush injury | Aortic dissection |
Burns (extensive) | Peritoneovenous shunt |
Extracorporeal circulation (e.g., ECMO) | Intra-aortic balloon pump |
Pregnancy catastrophes |
|
Placental abruption |
|
Amniotic fluid embolus |
|
Septic abortion |
|
Embolism of fat or cholesterol |
|
Hepatic failure (severe) |
|
Toxic/immune reactions (severe) |
|
Snake bites |
|
Hemolytic transfusion reactions |
|
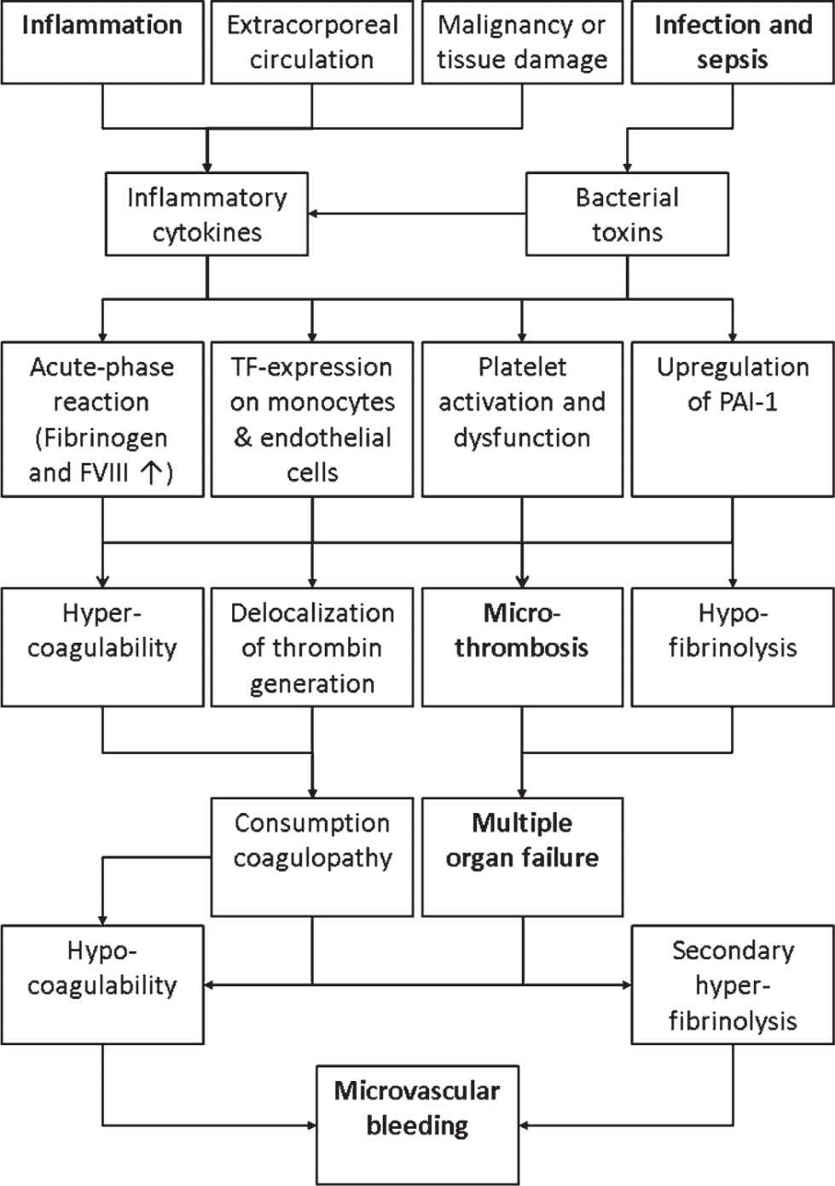
FIGURE 25.1 Pathophysiology of DIC is mainly triggered by inflammation, tissue/organ damage, infection, and sepsis, finally resulting in microthrombosis, multiple organ failure, and microvascular bleeding.
2. Clinical features of DIC include hemorrhage from operative or traumatic wounds, oozing from venipuncture sites, petechiae, and ecchymosis. Micro and macro vascular thrombosis can lead to organ failure.
3. Diagnosis includes clinical and laboratory data. Recommendations include the use of a DIC scoring system, of which three exists using different criteria for different types of DIC.
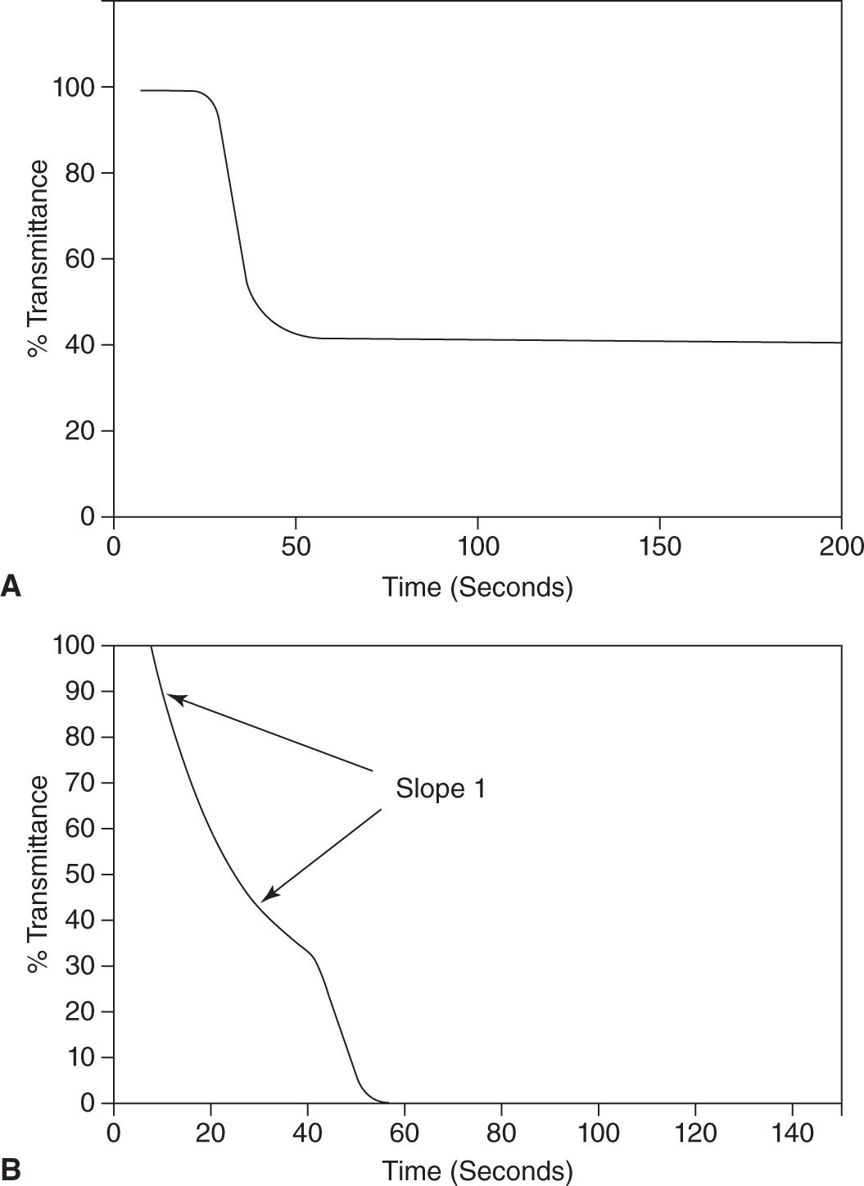
4. Laboratory tests such as prolonged PT and INR, reduction of platelet counts, and reduced fibrinogen and antithrombin (AT) levels may overlap with other coagulopathies. Elevation of fibrin-related markers such as D-Dimer, fibrin degradation products, and soluble fibrin are common findings not specific to DIC. Peripheral blood smears can reveal schistocytes (fragmented red blood cells), which are formed as red blood cells flow through fibrin stands in the microvasculature are severed. Point-of-care testing includes thromboelastometry/thromboelastography (ROTEM/TEG) and PTT waveforms to support the diagnosis (Fig. 25.2).
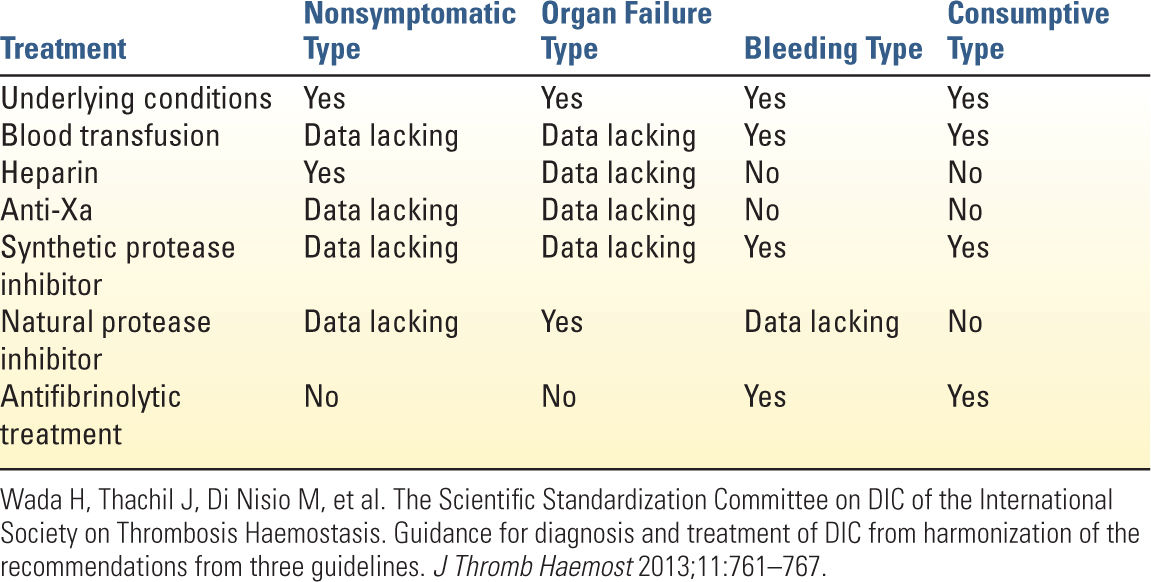
5. Treatment of DIC is aimed at correcting the underlying cause, blood product transfusion when indicated, and pharmacologic treatment (Table 25.2). Blood products should be transfused to correct for active bleeding or in preparation for life-saving procedures. Fibrinogen levels should then be corrected to 150 to 200 mg/dL. Platelet transfusion should be considered in patients who are actively bleeding and who have a platelet count of <50,000/mm3. However, platelet transfusion should be considered carefully since it may aggravate multiple organ failure and result in secondary bacterial infections. In nonbleeding patients, the threshold for platelet transfusion should be 10,000–20,000/mm3. Other blood components such as FFP and PRBC should be transfused only in patients with active bleeding or at high risk of bleeding.
a. Pharmacologic treatment depends on the type of DIC and includes anticoagulation or antifibrinolytics. The balance of coagulation and fibrinolysis can be further characterized with point-of-care testing (ROTEM/TEG and whole-blood impedance aggregometry) to guide treatment, but hematologic consult should be considered in complicated cases of DIC.
B. Liver Disease affects coagulation as the majority of factors, except factor VIII and von Willebrand’s factor (vWF), are produced in the liver. In chronic liver disease, coagulation factors (I, II, V, VII, IX, X, and plasminogen) and inhibitors (antithrombin, protein C and S, α2-antiplasmin, as well as the vWF-cleaving enzyme ADAMTS13) synthesized by the liver are decreased while the vascular endothelium-derived factor VIII, vWF, tPA, and PAI-1 are elevated (Fig. 25.3). Clinically, this results in a rebalanced hemostasis, though standard plasmatic coagulation tests such as PT and PTT may be elevated. In fact, thrombin generation assays containing thrombomodulin and viscoelastic tests (thromboelastography and thromboelastometry) demonstrate that patients with long-standing liver disease tend to be hypercoagulable. They should be considered for thromboprophylaxis unless contraindicated. Similarly, in acute liver failure, overt bleeding is less common than would be expected due to a “rebalanced” coagulation dysfunction. Thrombocytopenia frequently occurs as the result of splenic sequestration but may be compensated by high vWF levels.
1. Prophylactic transfusion of FFP and platelets should be avoided, and hemostatic interventions should only be performed in case of clinically relevant bleeding. There is usually response to vitamin K supplementation. Antifibrinolytic drugs or coagulation factor concentrates such as fibrinogen, prothrombin complex concentrate (PCC), or activated recombinant factor VII (rFVIIa) may be appropriate in certain patients. However, the potential benefit of improving hemostasis at the expense of increasing the thrombotic risk should be carefully evaluated in individual patients. Here, viscoelastic testing (ROTEM/TEG) seem to be helpful to guide therapy in bleeding patients. Notably, procoagulant agents such as rFVIIa have been shown to improve laboratory values such as PT, without improving control of bleeding during liver transplant or upper GI hemorrhage.
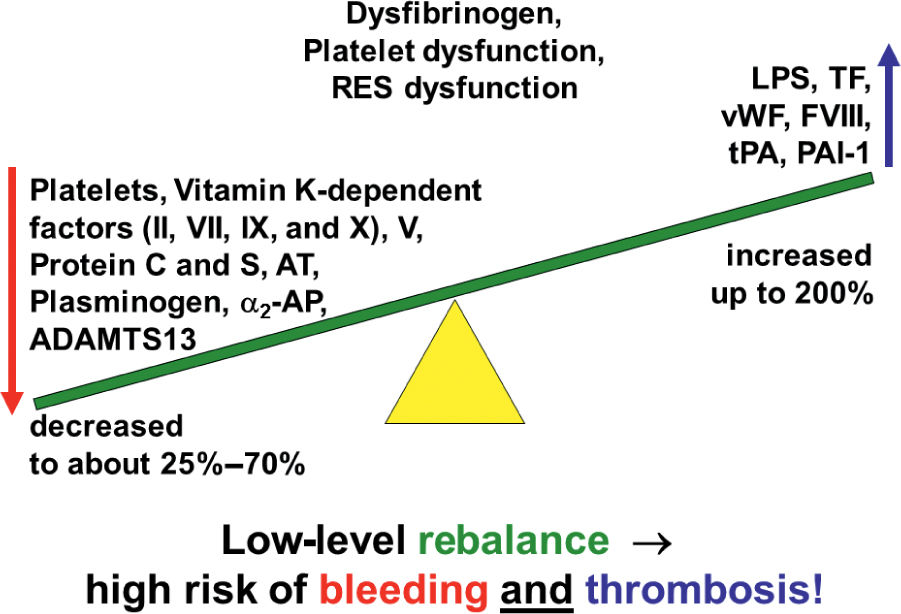
FIGURE 25.3 Coagulopathy in cirrhosis. In cirrhosis, hemostasis is rebalanced at a low level associated with a high risk of bleeding and thrombosis.
C. Vitamin K Deficiency. Vitamin K, which is produced in the intestinal mucosa, is important in the synthesis of coagulation factors in the liver. These factors include the clotting factors II, VII, IX, and X as well as the anticoagulant factors protein C and protein S. Vitamin K deficiency is common in prolonged illness and can be repleted 2.5 to 25 mg subcutaneously once or 10 mg subcutaneously daily for 3 days. Vitamin K can be given intravenously, though with the risk of anaphylaxis. Oral vitamin K in the critically ill patient depends on ability to have enteric medication and adequate absorption. Furthermore, the activity of vitamin K-dependent coagulation factor is low in patients under oral anticoagulation with coumarins depicted by an increased INR (targeted range 2–3.5). In severe and life-threatening bleeding due to vitamin K-antagonists (VKAs), for example, in intracerebral hemorrhage or GI bleeding, the effect can be reversed rapidly by the administration of four-factor PCCs. Four-factor PCC is FDA-approved for this indication since 2013. Whether four-factor PCCs are useful or not to reverse the effect of new oral anticoagulants (NOACs: dabigatran, rivaroxaban, apivaban, and edoxaban) is still under debate.
D. Uremia. Abnormal platelet function in uremia may contribute to significant bleeding in the trauma or perioperative settings. The primary therapy is hemodialysis and should be strongly considered prior to invasive procedures in the event of uremia-related coagulopathy. IV desmopressin as a slow infusion increases multimers of factor VIII: vWf. Cryoprecipitate and treatment of anemia with transfusion of PRBC in the acute setting can be considered, as well. Therapy with less immediate response time includes conjugated estrogens (over the course of 4–7 days) and erythropoietin (over the course of weeks to months).
E. Trauma-Induced Coagulopathy (TIC) or Acute Coagulopathy of Trauma Shock (ACoTS) is accepted to be a discrete clinical entity different from DIC (Fig. 25.4). In contrast to DIC hypoperfusion-induced activation of protein C with subsequent cleavage of activated factors V and VIII and down-regulation of PAI-1 result in endogenous anticoagulation and primary hyperfibrinolysis. Furthermore, shedding of the endothelial glycocalyx leads to liberation of heparinoids, which intensifies endogenous anticoagulation. In addition, TIC is modulated by hemodilution, hypothermia, and acidosis. TIC is functionally characterized by a reduction in clot strength. With a threshold of clot amplitude at 5 minutes of ≤35 mm, rotational thromboelastometry can identify acute traumatic coagulopathy at 5 minutes and predict the need for massive transfusion. Therefore, early administration of tranexamic acid and fibrinogen replacement is crucial in treatment of TIC. Fibrinogen levels should be corrected to at least 150 to 200 mg/dL. Concepts to treat TIC vary widely between the United States and Europe, using a fixed transfusion ratio of packed red blood cells, FFP, and platelets on the one hand and an individualized goal-directed bleeding management using coagulation factor concentrates (fibrinogen and four-factor PCC) guided by viscoelastic testing (ROTEM/TEG) on the other hand. RCTs are missing to show which approach is superior in severe trauma. In traumatic brain injury (TBI), the risk of bleeding as well as thrombosis is high. A neurointensivist should be involved in the care of a critically ill patient with significant head trauma.
F. Postoperative Bleeding, for example, after cardiac surgery or liver transplant, usually is multifactorial. Here, hyperfibrinolysis, fibrinogen deficiency, fibrin polymerization disorders, thrombocytopenia, thrombocytopathy, and impaired thrombin generation can play a major role. Standard plasmatic coagulation tests are limited due to their long turn-around time and their inability for predicting bleeding and guiding hemostatic therapy in the perioperative setting. Here, ROTEM/TEG as well as point-of-care platelet function analysis have been shown to be superior in reducing transfusion requirements, transfusion-associated adverse events, thromboembolic events, and improving patients’ outcomes. The use of perioperative bleeding management algorithms guided by ROTEM/TEG are highly recommended here (Fig. 25.5). Their clinical and cost effectiveness has been proven in several studies and health technology assessments.
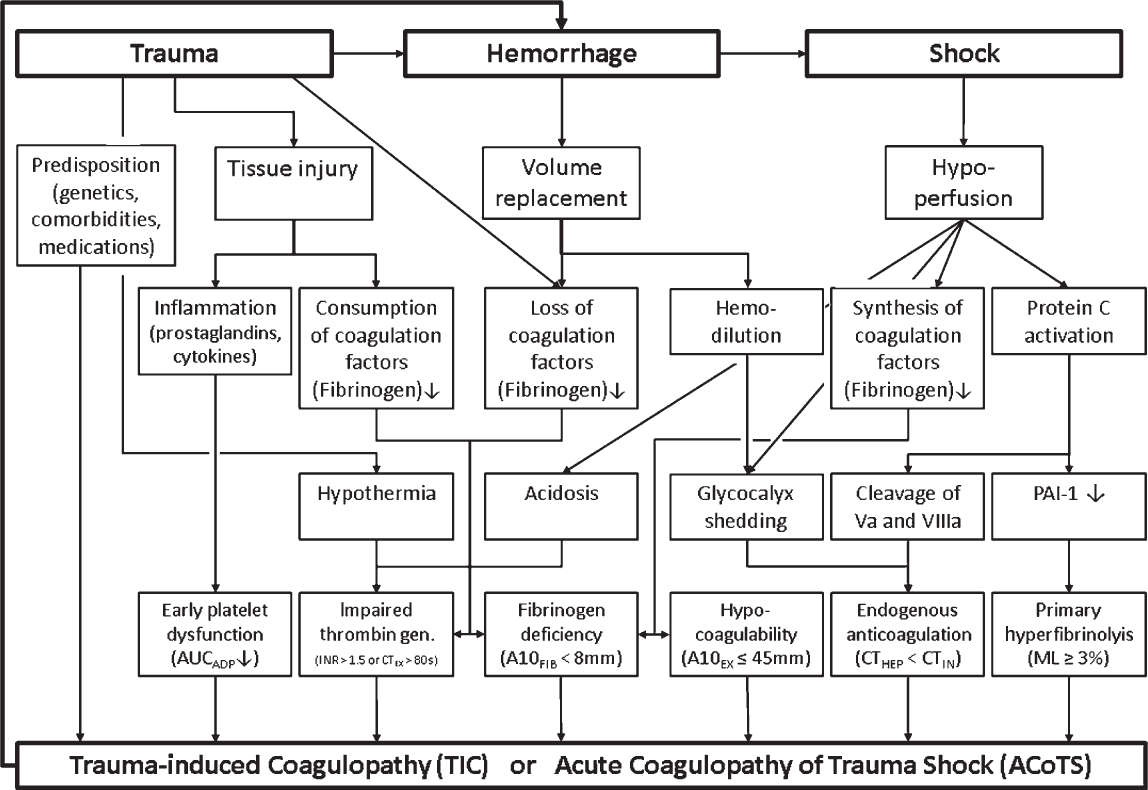
FIGURE 25.4 Pathophysiology of TIC and corresponding triggers of thromboelastometry and whole-blood impedance aggregometry; A10, amplitude of clot firmness 10 minutes after CT; AUC, area under the curve; CT, coagulation time; EX, extrinsic thromboelastometry (EXTEM); FIB, fibrin thromboelastometry (FIBTEM); HEP, heparinase modified thromboelastometry (HEPTEM); IN, intrinsic thromboelastometry (INTEM); INR, International normalized ratio; ML, maximum lysis.
G. Clotting
1. Hypercoagulability abnormalities include congenital conditions such as factor V Leiden (activated protein C resistance), prothrombin mutation, protein C and S deficiencies, antithrombin deficiency, antiphospholipid antibodies (lupus anticoagulant [LA]), and hyperhomocysteinemia. Evaluation includes thorough history and labs, supported by genetic testing for the patient and family members. However, in the setting of acute illness and elevation of acute-phase reactants, these tests may not be specific. Expert hematologic consultation is recommended in critically ill patients.
a. Medications such as oral contraceptives and smoking or obesity and diabetes should be taken into consideration when critically ill patients with hypercoagulability are evaluated. Pregnancy, trauma, and surgery predispose and contribute to the multifactorial process of hypercoagulability.
b. Treatment is individually tailored. Compression stockings, sequential compression devices, and prophylactic and therapeutic anticoagulation are determined on the basis of history and clinical setting.
2. Heparin-induced thrombocytopenia (HIT) is classified as nonimmune mediated (HIT type 1) or immune mediated (HIT type 2). HIT type 1 is a benign fall in platelet count, usually within 5 days of initiating heparin. The platelet count usually does not fall below 100,000 mm3 and heparin does not have to be discontinued or avoided in the future.
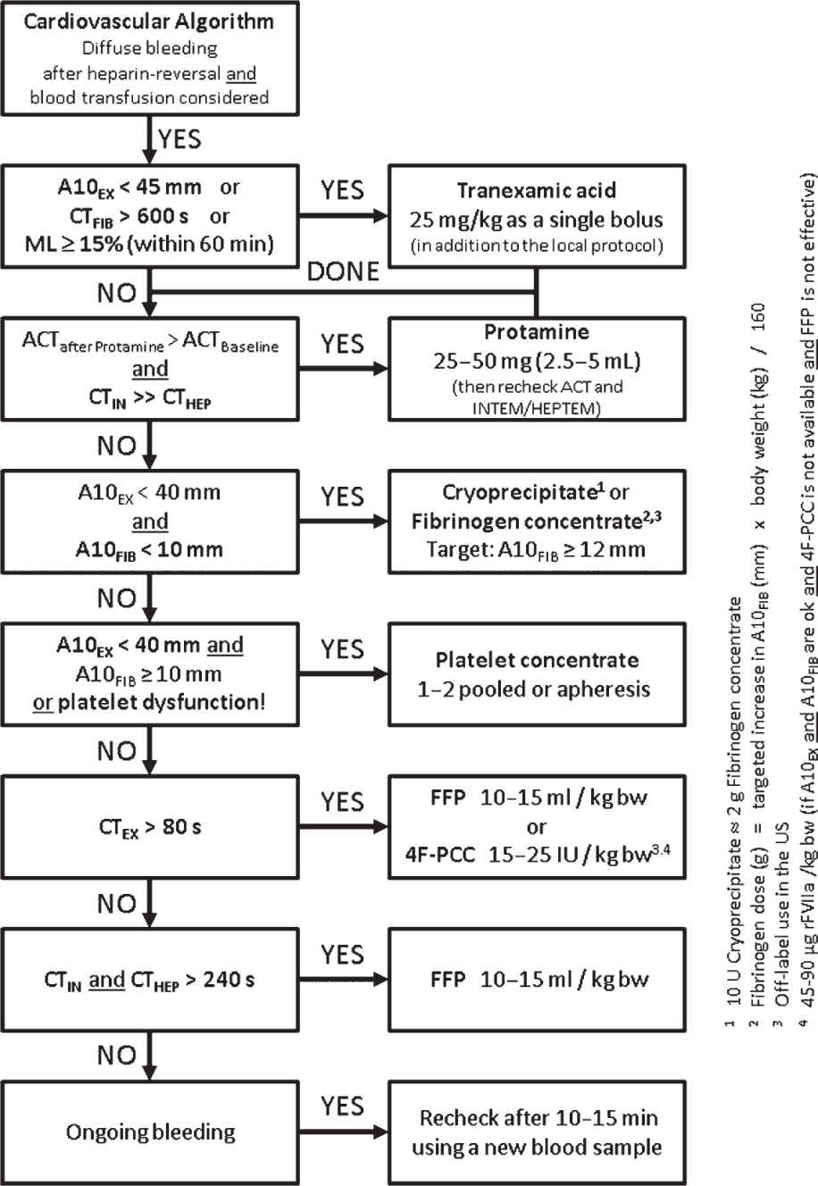
FIGURE 25.5 Point-of-care algorithm for bleeding management in cardiovascular surgery guided by thromboelastometry and whole-blood impedance aggregometry; 4F-PCC, four-factor prothrombin complex concentrate; A10, amplitude of clot firmness 10 minutes after CT; ACT, activated clotting time; bw, body weight; CT, coagulation time; EX, extrinsic thromboelastometry (EXTEM); FIB, fibrin thromboelastometry (FIBTEM); HEP, heparinase modified thromboelastometry (HEPTEM); IN, intrinsic thromboelastometry (INTEM); ML, maximum lysis; rFVIIa, activated recombinant factor VII.
a. HIT type 2 is immune mediated. IgG antibodies are formed against heparin-platelet factor 4 (PF-4) complexes. This results in platelet activation and aggregation, leading to pathologic platelet aggregation, thrombocytopenia, and vascular thrombosis. HIT antibodies binding to endothelial cell surfaces may result in tissue factor expression and a prothrombotic state.
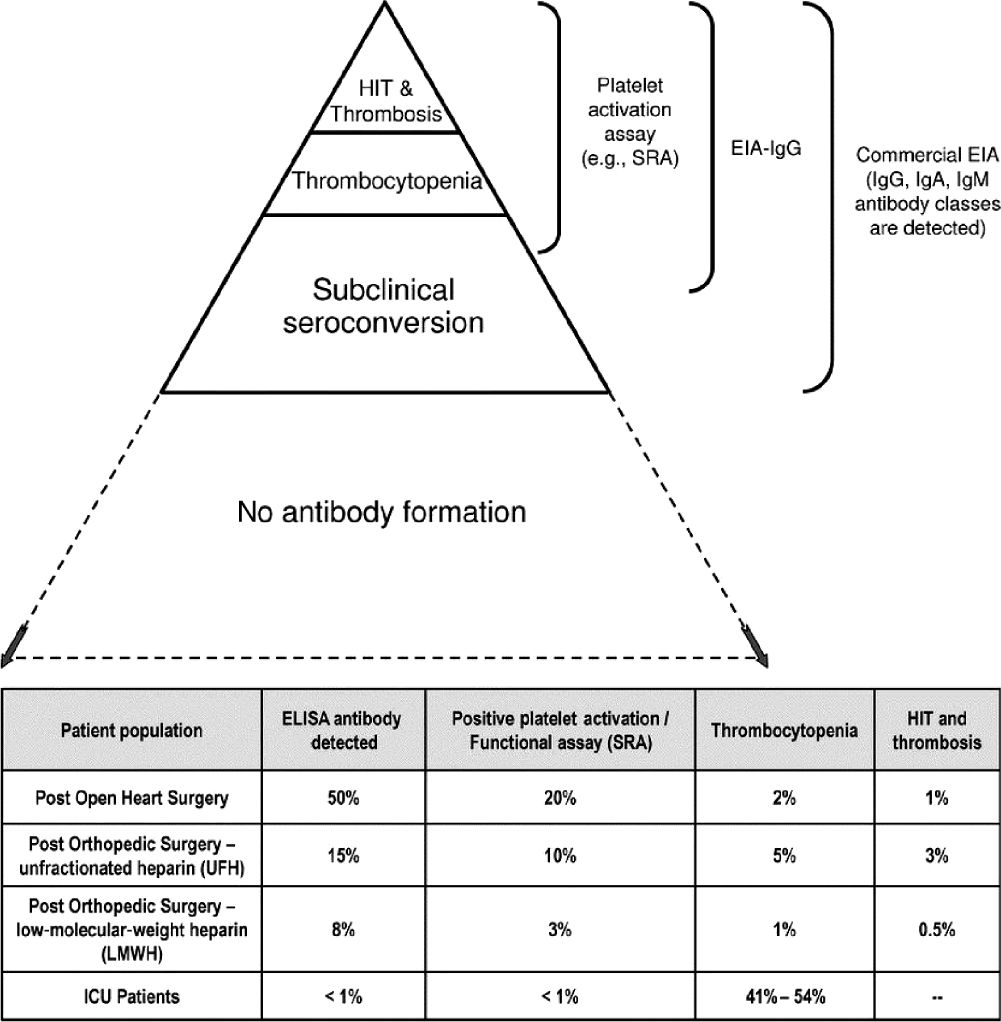
b. Up to half of cardiac surgery patients and 15% of orthopedic surgery patients develop HIT type 2 by immunologic assays (ELISA). However, only 1% to 3% of these patients develop clinically significant HIT type 2. This can be significantly reduced by use of low-molecular-weight heparin (LMWH) and eliminated with fondaparinux or direct thrombin inhibitors (DTIs) such as argatroban (Fig. 25.6). Argatroban is approved for prophylaxis and treatment of thrombosis in patients with or at risk of HIT. In patients with hepatic impairment or multiple organ failure, the argatroban dosage should be decreased to 0.1 to 0.2 µg/kg/min in order to avoid bleeding complications. Argatroban therapy can be monitored by aPTT or ecarin clotting time (ECT). Since aPTT reaches a plateau at higher argatroban plasma concentrations, an overdose may not be recognized. Therefore, ecarin-based assays are more reliable for monitoring DTIs.
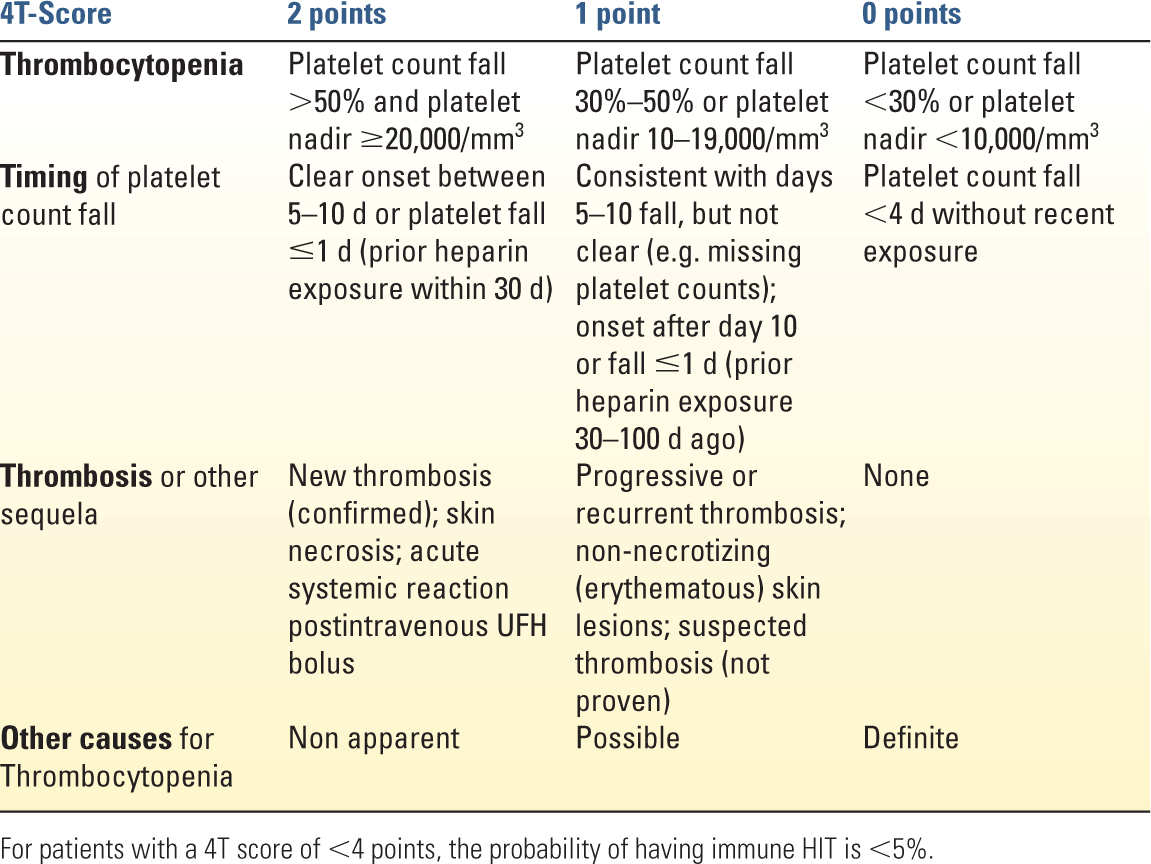
c. Diagnosis is made by history and physical, as well as a decrease in platelet count by 50% from baseline (but usually not less than 50,000 mm3) within 5 to 14 days of heparin exposure. Previous heparin exposure can lead to a quicker decrease in platelets. Platelet recovery after ceasing exposure to heparin and tachyphylaxis or resistance to heparinization is also suggestive. The clinical 4T-score can be used to estimate the probability of HIT type 2 (Table 25.3). For patients with a 4T-score of less than 4 points, the probability of having immune HIT is less than 5%. Platelet serotonin release assays (SRAs) are more specific than ELISA assays, but take longer to obtain. Whole-blood impedance aggregometry can be used as a functional assay for platelet-activating HIT-antibodies with a similar sensitivity and specificity compared with SRAs but with a shorter turn-around time. A high index of suspicion in the correct clinical setting (4T score ≥4 points) should be treated as positive for HIT type 2.
d. Treatment involves discontinuing all exposure to heparin, including heparin-coated catheters, flushes, hemodialysis, or extracorporeal circuitry. Appropriate anticoagulation is essential as 50% to 75% of patients with HIT type 2 develop thrombotic complications. Appropriate anticoagulation is patient specific and would include direct thrombin inhibitors such as argatroban. Platelet transfusion should be restrictive due to thrombotic concerns. Longer-term anticoagulation (at least 6–8 weeks) will generally be required.
3. Sickle cell disease in African Americans are caused by substitution of the amino acid valine for glutamic acid on the β-chain of hemoglobin. Clinical presentation occurs with homozygotes. Similar presentations can occur for homozygotes of SC or β-thalassemia. Heterozygote carriers usually do not present clinically.
a. Hypoxia, hypothermia, ischemia, acidosis, and hypovolemia can cause a “sickling” deformity of the red blood cell. Resultant microvascular obstruction can cause tissue ischemia and infarction. Sickle cell crisis present with nonspecific signs such as fever, leukocytosis, and tachycardia as well as signs of end-organ dysfunction. Significant anemia can occur due to the shortened lifespan and destruction of the red blood cells. Treatment involves addressing the precipitating causes, providing adequate pain relief, and transfusion only when indicated.
H. Frequent Hematologic Diseases
1. Hemophilia A is a hereditary disorder of factor VIII and hemophilia B is a hereditary disorder of factor IX. History and physical findings are supported by laboratory findings of an elevated intrinsic pathway clotting time (PTT), with a normal PT/INR. Platelet function is normal but the blood clot is unable to be stabilized, so bleeding will recur. Treatment includes factor VIII, factor IX, cryoprecipitate, and desmopressin (DDAVP). rFVIIa and activated PCC (FEIBA = factor eight inhibitor bypassing activity) are indicated in patients with acquired hemophilia due to inhibitors. Expert hematology consultation is recommended in critically ill patients.
2. Von Willebrand’s disease is a primarily autosomal-dominant genetic disorder resulting from defects in vWF. vWF normally anchors platelets to collagen while strengthening clotted platelets and stabilizing factor VIII. Treatment includes DDAVP, human factor VIII/vWF complex concentrates, and cryoprecipitate preferably over FFP. In certain patients with acquired von Willebrand’s disease, high-dose intravenous gamma globulin has been used successfully. Again, expert hematology consultation is recommended in critically ill patients.
II. DIAGNOSIS
A. Physical Exam and careful history are critical in diagnosing the coagulopathy and cause. Early investigation should include a thorough review and assessment for therapeutic agents contributing to the coagulopathy. Current nutritional status, history of bleeding in the past, and nonmedication causes of coagulopathy should also be assessed. In critically ill patients, concurrent illnesses such as sepsis, multiple organ failure, recent heparin exposure, and mechanical circulatory device requirements are just a few of many contributors to coagulation pathology.
1. The pattern of bleeding helps differentiate between diagnoses that may present with similar laboratory findings. Major vascular sources of bleeding as opposed to petechiae and oozing from vascular catheter sites and mucosal surfaces point to markedly different causes and treatments.
B. Standardized Questionnaire on Bleeding and Drug History
Most of the preexisting hemostatic disorders, resulting in unexpected perioperative bleeding, are impairments of primary hemostasis, for example, von Willebrand’s disease or drug-induced platelet dysfunction. Therefore, these patients cannot be identified by standard plasmatic coagulation tests, such as aPTT and PT, but by a standardized questionnaire on bleeding and drug history, complemented by platelet function analysis, if applicable. Notably, not only antiplatelet drugs, such as COX inhibitors, ADP receptor blockers, and glycoprotein IIb/IIa receptor antagonists, can inhibit platelet function but NSAIDs, antibiotics, cardiovascular and lipid-lowering drugs, antidepressants, volume expanders, radiographic contrast agents, as well as chemotherapeutic agents can also result in acquired platelet function disorders. This has to be considered, in particular if standard plasmatic coagulation tests and viscoelastic testing (ROTEM/TEG) show normal results in bleeding patients.
C. Activated Partial Thromboplastin Time (aPTT) is performed by adding an activator of the intrinsic pathway, for example, ellagic acid or silica, phospholipids, and calcium to a citrated plasma sample. Normal values of aPTT vary widely depending on the reagent and analyzer used. There is an increasing number of heparin- and lupus anticoagulant–sensitive reagents on the market. The test is sensitive to decreased amounts of coagulation factors of the intrinsic pathway (prekallikrein), factors XII, XI (hemophilia C), IX (hemophilia B), and VIII (hemophilia A), the common pathway (factors X, V, II, and fibrinogen), unfractionated heparin (UFH), direct thrombin inhibitors (DTIs), antibodies to factor VIII or IX (acquired hemophilia), and antiphospholipid antibodies such as lupus anticoagulant (LA), generated in some anti-immune diseases. Antiphospholipid antibodies prolongs aPTT by reducing the availability of phospholipids—required for coagulation—in plasmatic coagulation tests (in vitro). Notably, aPTT prolongation due to an antiphospholipid syndrome (APS) is not associated with bleeding but with thrombosis. In vivo, antiphospholipid antibodies bind to phospholipids and lipoproteins in the cell membrane of platelets, resulting in platelet activation (similar to HIT) and arterial and venous thrombosis. aPTT can be used to guide unfractionated heparin (UFH) therapy but is not sensitive enough to LMWH. Here, anti–factor Xa assays have to be used if monitoring is required. Since aPTT reaches a plateau at higher DTI plasma concentrations, an overdose may not be recognized. The clinician should be aware that the positive and negative predictive value for bleeding is low for aPTT, which means that neither a prolonged aPTT has to be associated with bleeding (e.g., in APS) nor a normal aPTT excludes bleeding. Correction of an abnormal aPTT in surgical patients is not always indicated unless the patient is bleeding. Notably, an abnormal, biphasic aPTT waveform recorded during optical aPTT measurement (Fig. 25.2) can be detected in the early phase of sepsis and DIC and is predictive for high mortality. It can be used to discriminate between systemic inflammatory response syndrome (SIRS) and sepsis with a high sensitivity and specificity. Furthermore, thromboelatometric lysis index (LI60) using a cut-off value of ≤3% is superior to blood procalcitonin levels in discriminating between postoperative patients with SIRS and sepsis.
D. Anti–Factor Xa Assays are a chromogenic assays that facilitates the measurement of inhibition of factor Xa by UFH, LMWH, fondaparinux, and direct Xa inhibitors (DXaIs), such as rivaroxaban and apixaban. Since LMWH, fondaparinux, and DXaIs do not or even minimally prolong aPTT, anti–factor Xa assays have to be used if therapeutic levels of these drugs have to be monitored. Furthermore, the aPTT cannot be used to monitor UFH in some instances, for example, in the presence of antiphospholipid antibodies or in case of factor XII deficiency. In these cases, monitoring of UFH with anti–factor Xa assays is more appropriate.
E. Prothrombin Time (PT) is performed by adding thromboplastin (tissue factor = factor III), as the activator of the extrinsic pathway, phospholipids, and calcium to a citrated plasma sample. The test is sensitive to decreased amounts of coagulation factors of the extrinsic (factor VII) and common pathway (factors X, V, II, and fibrinogen). Since most vitamin K–dependent coagulation factors (II, VII, IX, and X) are involved in the extrinsic and common pathway (except factor IX), PT has been designed for monitoring of VKAs (coumarins), such as warfarin. However, thromboplasin reagents used for PT measurement vary widely regarding their tissue factor activity, in particular between the United States and Europe. Therefore, the international normalized ratio (INR) has been instituted in 1983 by the WHO in order to permit comparability of results in warfarin-treated patients between different laboratories. Therefore, warfarin therapy can be guided by a targeted INR value independent from the performing laboratory. The INR is the result of the PT-ratio (PTpatient/PTcomtrol) corrected by a thromboplastin reagent-specific international sensitivity index (ISI). The ISI value of the WHO standard thromboplastin reagent is 1. Most thromboplastin reagents used in Europe have an ISI value close to 1. In contrast, US thromboplastin reagents have an ISI value of 2.0 to 2.6. The majority of PT reagents contain the heparin-neutralizing agent polybrene, a positively charged polymer, in order to eliminate the effect of UFH. This allows for better discrimination between heparin and warfarin effects since heparin prolongs aPTT only, and warfarin prolongs PT and increases INR only. Although the INR is frequently used to assess coagulation impairment in patients with trauma and liver disease (e.g., to verify TIC or to calculate MELD score), it may not be valid here. In liver disease, vitamin K–dependent coagulation factors are low but compensated by high factor VIII levels and a decrease in the activity of the vitamin K–dependent anticoagulants protein C and S. This is not reflected by PT and INR because it is dependent on the presence of thrombomodulin. Accordingly, endogenous thrombin potential (ETP) of plasma samples from patients with cirrhosis is lower compared with plasma from noncirrhotic patients. However, the addition of thrombomodulin, which allows for activation of protein C, results in equalization of ETP between patients with and without cirrhosis, demonstrating a rebalance of hemostasis. Therefore, PT and INR values in patients not treated with warfarin have to be interpreted carefully in the perioperative setting.
F. Thrombin Time (TT) is performed by adding thrombin to a citrated plasma sample. The test is sensitive to fibrinogen deficiency, dysfibrinogenemia, fibrin(ogen) degradation products, fibrinolysis, heparin, and direct thrombin inhibitors (DTIs), such as hirudin, argatroban, bivalirudin, and dabigatran. The increasing use of DTIs led to a renaissance of the TT, in particular in its modification as plasma-diluted TT (dTT). For the dTT assay, patient plasma is diluted 1:3 to 1:4 with normal plasma before performing the TT assay, and the results are compared with a DTI-specific calibration curve. Notably, none of the global coagulation tests aPTT, PT, or TT is sensitive to factor XIII deficiency.
G. Ecarin Clotting Time (ECT) is performed by adding ecarin to plasma or whole blood. Ecarin converts prothrombin to meizothrombin, an intermediate product of thrombin generation. Meizothrombin has a lower enzymatic activity compared with thrombin but forms 1:1 complexes with hirudin and other DTIs such as argatroban, bivalirudin, and dabigatran (see section III.B.5). ECT is not influenced by heparin and shows a linear correlation to DTI concentration in a wide range. A whole-blood ECT assay (ECATEM) is also available for the ROTEM device but only licensed in Europe, yet.
H. Fibrinogen is the first factor dropping down to a critical level in case of severe bleeding. The normal range for fibrinogen is 150 to 400 mg/dL and in the third trimester of pregnancy 450 to 600 mg/dL. In acute-phase reaction, it can rise to about 1,000 mg/dL. In severe bleeding, it is crucial to maintain the plasma fibrinogen level above 150 to 200 mg/dL. Therefore, the historic cutoff value of 100 mg/dL for fibrinogen substitution seems not to be adequate in severe bleeding. FFP is not effective to reach this target. Here, transfusion of cryoprecipitate or fibrinogen concentrate is much more effective. Fibrinogen levels can be measured by several methods. The Clauss fibrinogen assay is a modified TT assay using prediluted plasma and high thrombin concentrations. The PT-derived fibrinogen assay is using the plateau of the light transmission curve to calculate plasma fibrinogen concentration. All optical methods are influenced by infused colloids resulting in wrong high fibrinogen values by 15% to 90%, depending on the reagent used. In contrast, viscoelastic testing using the FIBTEM (ROTEM) or functional fibrinogen (FF) assay (TEG) demonstrate impaired fibrin polymerization after colloid infusion, associated with increased blood loss and transfusion requirements. Notably, TEG FF assay result in higher values compared with ROTEM FIBTEM assay since platelets are incompletely blocked in the FF assay. This difference should be considered in interpretation of these functional fibrinogen test results.
I. Fibrin Monomers (FM) are produced from the action of thrombin on fibrinogen without cross-linking of the fibrin monomers by factor XIII. FMs are elevated in DIC. Furthermore, increased FM concentration can be a sign of decreased factor XIII availability.
J. Fibrin(ogen) Degradation Products (FDPs) are peptides produced from the action of plasmin on fibrinogen or on fibrin monomers. They are measurable by serum assays and may aid the diagnosis of primary fibrinolysis or DIC. Furthermore, FDPs modulate clotting assays by interfering with fibrin monomer polymerization and by impairing platelet function. FDPs are often elevated in cirrhosis due to impaired clearance from circulation.
K. D-Dimer is a specific fragment produced when plasmin cleaves cross-linked fibrin and can be measured by a serum assay. Almost all patients with acute venous thromboembolism (VTE) present with an elevated D-dimer level. However, elevated D-dimer is not specific for VTE since it can be associated with several other conditions, including DIC, postsurgical or posttraumatic patients, pregnancy, and malignancy. However, a normal D-dimer test usually excludes the diagnosis of VTE.
L. Single Factor Assays are specialized tests quantifying the activity of individual coagulation factors. They can be used to clarify prolonged global plasmatic coagulation tests, such as aPTT, PT, and TT. On the one hand, isolated prolongation of aPTT is suspicious for hemophilia A (factor VIII), B (factor IX), or C (factor XI), but can be prolonged significantly in factor XII deficiency, too, which is not associated with bleeding. On the other hand, isolated prolongation of PT can be a sign of factor VII deficiency. Notably, factor XIII deficiency is not associated with a prolongation of aPTT, PT, or TT, but can be the cause for unexpected postoperative bleeding. Single-factor analyses are usually performed in concert with a clinical pathology or hematology consultation.
M. Activated Clotting Time (ACT) is a clotting test in which kaolin, celite, or glass beads are added to a noncitrated whole-blood sample to activate the intrinsic pathway. ACT usually is performed as a point-of-care test in the acute setting, for example, during cardiopulmonary bypass or ECMO, to monitor high heparin concentrations where aPTT cannot be measured any more (>180 s). Since ACT is a nonspecific whole-blood test, it cannot only be influenced by heparin and protamine but also by a lot of other variables, such as hemodilution, fibrinogen, platelets, aprotinin, and glycoprotein IIb/IIIa receptor antagonists. This is important for a meaningful interpretation of ACT test results. Since normal ACT depends on the test system used, it should be standardized by an institution.
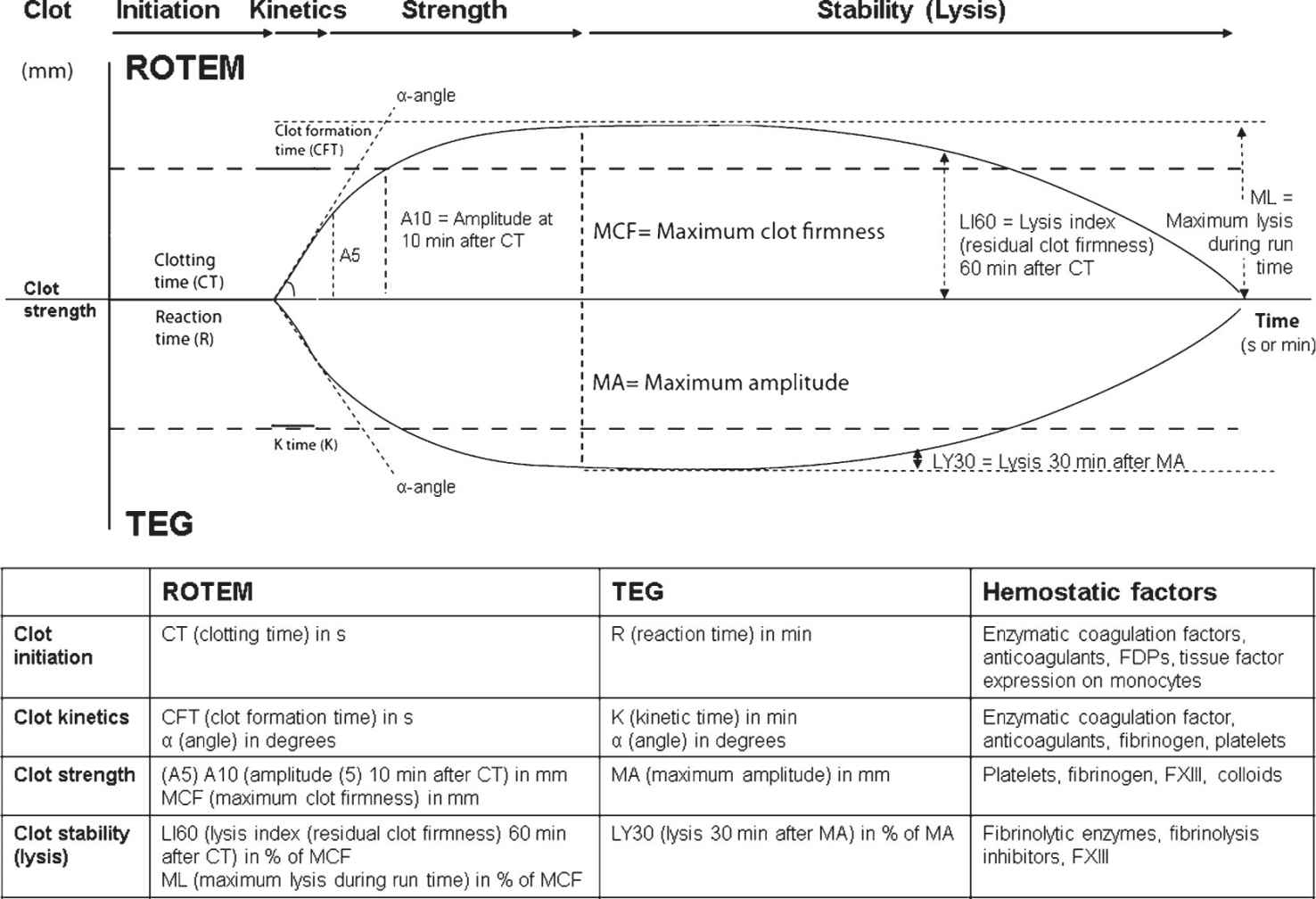
N. Thromboelastometry (ROTEM)/Thromboelastography (TEG) are viscoelastic test devices used most often at the point-of-care in the emergency room (ER), the operating room (OR), or in the ICU. Both devices are assessing the change in viscoelasticity of a small amount of whole-blood activated by different agents in a system where a heated cup and a pin are oscillating against each other. Viscoelastic testing provides real-time, dynamic information about the whole coagulation process, including clot initiation (thrombin generation), clot kinetics, clot strength, and clot stability (lysis). However, it does not detect the effects of antiplatelet drugs, such as aspirin or clopidogrel, since platelets are activated via the thrombin receptor pathway here. Therefore, viscoelastic testing often is combined with point-of-care platelet function analysis if platelet dysfunction is expected (see Section II.P). Both devices differ in some technical points, definition of parameters, and reagents used. Therefore, results are not completely interchangeable between both devices. Due to the use of an automatic pipette, the stabilization of the pin movement by a ball-bearing, and the contact-free detection of the pin movement by a laser light-mirror-system, the ROTEM system is more user friendly, enables even mobile use in military field hospitals, and provides low inter- and intraoperator variability of test results in a multiuser environment, such as in the ER, OR and ICU environment. The definition of ROTEM/TEG parameters and their clinical relevance are displayed in Figure 25.7. Specific patterns of the trace are characteristic for several coagulation abnormalities (e.g., hyperfibrinolysis, fibrinogen deficiency, thrombocytopenia, factor deficiencies of the extrinsic and intrinsic pathway, heparin effects; see Fig. 25.8), assisting the clinician with the diagnosis and appropriate treatment. Here, the use of ROTEM/TEG-guided bleeding management algorithms is highly recommended (Fig. 25.5), and their clinical and cost-effectiveness has been proven in several studies and health technology assessments. In ROTEM, decision making is speeded up by using early amplitudes of clot firmness (A5 in Europe, A10 in the United States) to predict final clot firmness. Furthermore, the diagnostic performance can be improved by using test combinations compared with a monotest approach. Both devices use different activators and additives in their assays (see Table 25.4). Education is required in perioperative bleeding management and in technical training with these tests as well as test interpretation to obtain the expertise and judgment to improve patient outcomes and decrease transfusion requirements.

Full access? Get Clinical Tree