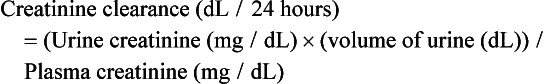56 Chronic kidney disease (CKD) is a common diagnosis in adult medicine.1–6 In order to properly identify patients at risk for adverse outcomes and disease progression, extensive work has focused on early and appropriate classification over the last 10 years. In particular, the National Kidney Foundation (NKF) proposed a classification system for CKD in 2002 using estimated glomerular filtration rate (eGFR) as a method to categorize patients.7,8 Table 56.1 describes current NKF guidelines of CKD. In regard to critical care, CKD patients have higher mortality rate following critical illnesses.9 Understanding this population’s physiology and underlying disease state may aid caregivers in attempting to improve this outcome. Table 56.1 As previously stated, currently CKD is staged in a five-tier system based on eGFR (see Table 56.1). The diagnosis relies on abnormal kidney function or urinalysis findings for 3 months or more. The serum creatinine as well as age, sex, gender, and race is used to generate an eGFR from a quadratic equation derived from the Modification of Diet in Renal Disease Study.7 Most CKD patients do not progress to end-stage renal disease (ESRD), however, because of the very high cardiovascular and noncardiovascular mortality rate associated with this at-risk population.10–13 Proposals exist to more clearly define elderly subjects with CKD stage 3 disease at risk for disease progression, though this practice has not become standard.14–16 Causes for CKD are broad and often multifactorial. Frequently, however, diabetes mellitus and hypertension play a major role. Hypertension may lead to kidney damage in the form of nephrosclerosis or chronic renal ischemia due to atherosclerotic vascular disease. An important recent development is the recognition that the higher prevalence of nondiabetic kidney disease in the African-American population may be due to genetic risk conferred by inherited variants in the apolipoprotein L1 gene (APOL1).17 Additional causes include immune-mediated glomerular diseases such as systemic lupus erythematosus, IgA nephropathy, membranous nephropathy, and other often pre-existing glomerulonephritides. Glomerulonephritis may be active as evidenced by hematuria and proteinuria or may have occurred in the patient’s past, leading to abnormal kidney function but a relatively bland urinalysis. Alternatively, primary tubulointerstitial diseases could exist owing to reflux nephropathy, sarcoidosis, chronic infections, allergic reactions, or side effects from medications such as cyclophosphamide (Cytoxan) and lithium. In general, the diagnosis of CKD should warrant a higher index of suspicion for concurrent, and potentially undiagnosed, cardiovascular disease (CVD). The CKD population is at risk for deterioration in kidney function during a hospitalization stay, particularly if a critical illness is present. Potential causative factors for this are listed in Box 56.1. Importantly, acute tubular necrosis may occur without obvious hypotension, and patients with reduced kidney function are more at risk for this complication.18 Intrinsic to the eGFR formula, as well as other estimates of renal function from the serum creatinine, is the presumption that a patient’s serum creatinine is stable. This finding may not be the case in ICU patients; as a result, imprecision should be expected in kidney function estimation in ICU patients.19 Serum creatinine is now recognized to potentially underestimate the level of renal function in a large population of patients, particularly those with low weight, small muscle mass, and liver disease.20 Additionally, both endogenous and exogenous factors can influence the measurement of serum creatinine.21–24 Box 56.2 describes situations in which serum creatinine measurements may not adequately reflect kidney function. Importantly, some vasoactive substances can impart a negative interference on creatinine values if the creatinine sample is obtained from a central line used for vasopressors.25 Nonetheless, creatinine variation has improved since values have been standardized to reference values.26,27 In general, current guidelines recognize the importance of stage 3 through 5 kidney disease for prognostication in outpatient and inpatient outcomes. Proteinuria is now associated with increased cardiovascular risk at all glomerular filtration rates (GFRs), including normal GFRs.28 Even mild kidney dysfunction, defined typically as a serum creatinine level over 1.5 mg/dL or GFR below 60 mL/minute, is associated with worse outcomes after coronary artery bypass graft (CABG), cardiac valvular, and general surgery.29–33 Concern exists about the accuracy of the Modification of Diet in Renal Disease (MDRD) equation at higher GFRs. For example, the newer creatinine-based CKD-EPI formula equation leads to a lower prevalence rate of CKD stage 3 than the MDRD equation, as more patients have an eGFR above 60 mL/minute.34–36 Measurement of serum cystatin C levels, a protein produced by all nucleated cells and freely filtered by the glomerulus, may provide more accurate estimates of kidney function than creatinine-based equations, particularly when kidney function is close to normal.37 Cystatin C may also provide prognostic information about future cardiovascular events.12 In fact, cystatin C has been proposed as a marker of “preclinical” kidney disease based on worse cardiovascular outcomes in elderly subjects with higher cystatin C levels.38 Despite a growing body of literature, use of cystatin C is not routine in clinical practice. In this equation, units will need to be converted from dL/24 hours to mL/minute by a conversion factor of 0.0694. If this measure is pursued, clinicians are counseled to pay close attention to units as well as the expectation that men excrete 1500 to 2500 mg/day of creatinine and women excrete 1000 to 1500 mg/day of creatinine.39 If a lower amount of urinary creatinine is obtained, the possibility of an undercollection should be considered. It is also worthwhile to note that historically, drug dosing has been determined by creatinine clearance and not eGFR. Over 80% of CKD patients with eGFR less than 60 mL/minute have hypertension.5 This has several important impacts on the management of ICU patients. First, longstanding hypertension, if uncontrolled, leads to adaptations in autoregulation. Autoregulation refers to the ability of blood vessels to constrict and dilate in the presence of hypertension and hypotension, respectively. Consequently, patients become acclimated to BPs near their “typical” BP and may not tolerate lower BPs despite these apparent lower values appearing in the normal range. A consequence of this may be hypoperfusion to the kidneys as well as the brain, heart, intestine, and other critical organs at BP levels that appear normal. If a history of poorly controlled hypertension is obtained, an astute clinician should aim to keep BP close to the level the patient being treated is used to. This very much requires individualized attention and thorough history taking. Second, CKD leads to sodium retention.40,41 The most notable ICU impact this will have is the potential for volume retention and the need for diuretics to manage hypertension in the CKD population.42–44 Several mechanisms lead to the development of hypertension in the CKD population. They include upregulation of the renin-angiotensin system, increased sympathetic nervous system activity, and impairment of endothelial-dependent arterial smooth muscle relaxation.45–51 Owing to the preceding mechanisms, CKD patients may develop more edema and volume overload with equivalent amounts of intravenous (IV) fluids. One worthwhile consideration, however, is that sodium retention associated with CKD may be counterbalanced by sodium loss from excessively high BP levels. This phenomenon, known as pressure natriuresis, may explain the rapid drops observed in BP in hypertension emergencies once excess sympathetic activity is controlled. Sodium homeostasis in patients with CKD is abnormal. With increased loss of sodium in the urine, but declining numbers of functioning nephrons, sodium balance is maintained.52 Though CKD patients are sodium “avid” and tend toward states of volume overload, they are less able to increase sodium retention in times of need and subsequently are also at risk for volume depletion. In comparison, subjects with normal renal function can quickly increase sodium absorption and avoid volume-depleted states.53 Tubulointerstitial disease and obstructive uropathy may be particularly at risk for episodes of volume depletion due to their recognized inability to increase sodium reabsorption in times of volume depletion.54 When volume overload is present, iatrogenic sources may be playing a role. Potential sources of sodium include maintenance IV fluids (including bicarbonate), antibiotics, and nutritional sources including total parenteral nutrition (TPN). Tracking fluid intake and output (“Is and Os”) does not clearly differentiate between the electrolyte makeup of the various sources. Consequently, volume overload may develop insidiously. When volume overload develops, the standard approach involves minimizing sodium-containing sources and initiating a diuretic regimen. Diuretics range from the more gentle thiazide diuretics, which are ineffective with a GFR below 30 mL/minute, to more potent loop diuretics. In general, if significant volume removal is desired, a diuretic regimen twice a day (every 12 hours) should be prescribed to avoid excess sodium reabsorption in the period after the major diuretic effect.55 Chronic administration of diuretics, prior to ICU admission, may lead to adaptive processes necessitating higher diuretic doses, combination therapy, or alternative approaches to volume overload states.56–59 Torsemide is the loop diuretic that may be closest to lasting 24 hours.60 Doses of loop diuretics need to be increased as renal function declines. Significant variability in loop diuretic kinetics exist; if a dose does not yield an increase in urine output within 2 hours, consideration should be given to increasing the dose. Alternatively, continuous infusion of loop diuretics may provide a better diuresis with less toxicity.61,62 Typically infusion rates of furosemide are 10 to 40 mg/hour. When a loop diuretic drip is ordered, a bolus should be given prior to initiating therapy to avoid a significant delay in efficacy. Diuretic failure is common in the CKD population. Potential explanations in an ICU population include acute kidney injury, inadequate dosing, and distal tubular hypertrophy due to chronic diuretic use. When high-dose loop diuretics do not achieve adequate diuresis, the addition of metolazone may improve urine output. Historically, metolazone is given 30 minutes prior to the loop diuretics, though it may be efficacious if given at other times as well. Finally, isolated ultrafiltration or potentially dialysis may be required for volume removal. Significant attention has been given to the importance of elevated intra-abdominal pressures leading to a reduction in renal function and, potentially, ability to respond to diuretics.63–68 Water concentration and dilution are abnormal in CKD patients. Normal patients can dilute and concentrate urine within a range of 40 to 1400 mOsm/kg.69 CKD leads to a narrower range of urine osmolality. Specifically, patients with advanced CKD have a urine osmolality much closer to 300 mOsm/kg; the ability to adjust urine osmolality declines as urine function worsens.70 Consequently, patients with CKD are prone both to hypernatremia and hyponatremia. Loop diuretics may further worsen the kidney’s ability to concentrate and dilute urine.71 The approach to hypernatremia and hyponatremia is similar to that in other patients. In hypernatremia cases, concurrent illnesses often lead to patients being unable to obtain water for themselves; a high urine output due to hyperglycemia or a high urea concentration may worsen this. Elevated urea, as with high serum glucose levels, functions as an osmolar agent in the tubular lumen and creates a state of relative antidiuretic hormone (ADH) resistance with the potential for substantial free water loss.72 Hypernatremia can lead to significant agitation and should be treated aggressively with increased water either intravenously, in the form of hypotonic IV fluids, or enterally. When severe, an estimation of free water deficit is appropriate. Unexplained polyuria with a low urine osmolality, hypernatremia, and CKD in an ICU patient with unclear history should warrant an investigation for previous lithium use.73 Hyponatremia reflects irregularities in water excretion in the vast majority of cases. CKD patients are less able to excrete water owing to the inability to lower urine osmolality.74 Sources of water include IV fluids, medications mixed in dextrose, and enteral sources. Patients often are not aware or not forthcoming in the amount of water they drink, though this is less an issue in ICU patients. The approach for most CKD patients is similar to that in other cases of hyponatremia, which involve looking for and reducing causes of increased water intake. Importantly, sodium abnormalities in the CKD patient, both with and without congestive heart failure, are associated with a higher mortality rate.75 Potassium (K+) regulation is an essential function of the kidneys in normal states. CKD patients routinely maintain serum K+ in the normal range despite losing up to 90% of renal function.76,77 Mechanisms underlying the maintenance of normal serum K+ in advanced CKD include increased K+ excretion per functioning nephron, increased gastrointestinal (GI) elimination of K+, and increased uptake of K+ by cells.78 Diabetes mellitus, however, may exacerbate the development of hyperkalemia due to the presence of a distal tubular acidosis (type 4) from aldosterone resistance. Hypokalemia may also be present due to current diuretic use or renovascular hypertension.79 Its presence is associated with a higher risk of death and future ESRD needs in the CKD population.80 ICU patients with CKD represent a population prone to irregularities in K+ homeostasis, in particular, hyperkalemia.81,82 Potential causes of hyperkalemia include insulin deficiency, hypoaldosteronism, and a loss of normal intestinal function, which plays a role in increased potassium excretion in CKD patients. Decreased blood flow to muscle and intestines in times of critical illness may impede potassium cellular uptake and excretion, respectively. Tissue ischemia from trauma, hypoxia, or tumor lysis is associated with an elevation in K+ which may occur quickly.83 One additional clinical consideration is that CKD patients who are fasting may develop surprising levels of hyperkalemia without any apparent cause due to low levels of insulin.84 Glucose-containing IV fluids may avoid this. The evaluation of hyperkalemia in CKD patients begins with assessing the cause. Typically, this includes close scrutiny of medications, IV fluids, and types of nutritional support. Frequently, ICU patients receive nutrition very different from what the body is used to and cannot manage a higher load of K+, especially in states of relative tissue perfusion. Importantly, many CKD patients are on diuretics as part of their routine outpatient medications; simply stopping these medications may lead to states of hyperkalemia. Hyperosmolality leads to a K+ shift out of cells caused by solvent drag; in its most common presentation, hyperglycemia, this can be reversed quickly with the use of insulin. Metabolic acidosis may be associated with hyperkalemia though organic acidosis states appear to cause less hyperkalemia than nonorganic acid–induced states.85 Medications are an important and growing cause of hyperkalemia. Congestive heart failure and proteinuric renal disease remain as two indications for possible dual renin-angiotensin-aldosterone blockade; hyperkalemia has been observed more frequently since the publication of the RALES trial.86 Box 56.3 lists some common causes of hyperkalemia in the CKD population and the associated mechanisms.87 The presence of hyperkalemia should warrant close attention to affected patients as its presence is associated with increased mortality rate.88
Chronic Kidney Disease
Introduction
Stage
eGFR
(mL/min/1.73 m2)
Urinalysis Findings
1
≥90
Hematuria, proteinuria, or imaging abnormalities at >3 months
2
60-89
Hematuria, proteinuria, or imaging abnormalities at >3 months
3
30-59
↑ or normal
4
15-29
↑ or normal
5
0-14
↑ or normal
Definition and Etiology
Diagnosis
Physiology
Hypertension
Electrolyte Disorders
Sodium
Water
Potassium