Fig. 29.1
Pre-stent angiogram
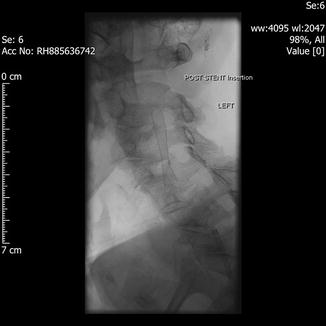
Fig. 29.2
Stent in situ
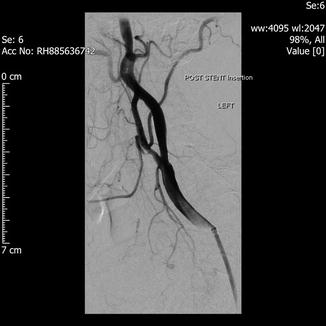
Fig. 29.3
Post stenting
Irrespective of treatment modality (open or endovascular), there is a per procedural stroke and death risk which needs to be balanced against the risk of further thromboembolic events in the patient who receives Best Medical Therapy alone. Given that the risk of events is so low for asymptomatic disease, the threshold for intervention must be much higher; conversely, the risk of further events after symptoms can greatly outweigh the risk of per procedural complications.
It is also a central tenet of treatment in symptomatic disease that endarterectomy does not reverse established ischaemic damage (or the related symptoms); it is an exercise in future risk reduction. Similarly, there is no indication for treating occlusive internal carotid disease as there can by definition be no thromboembolic risk through the occluded vessel.
Best Medical Therapy
The aspects of Best Medical Therapy (BMT) are well established: the cessation of cigarette smoking, statin therapy to lower LDL cholesterol, control of hypertension, glycaemic control in diabetes and the provision of antiplatelet therapy.
Questions to Be Answered
In consideration of a patient with internal carotid stenotic disease above the threshold for significance (50 % or 70 % dependent on the system used) and who is established on BMT, we have fundamental questions that need to be addressed in terms of guiding our management decisions: Is a patient with carotid disease better treated with Best Medical Therapy (BMT) or intervention plus BMT? Which is superior—carotid endarterectomy (CEA) or carotid artery stenting (CAS)? Does the presentation (symptomatic or asymptomatic carotid disease) influence the best therapy? What do we consider “Significant” in terms of treatment failure? Is it any stroke? Death? Stroke sub-types? Myocardial infarction? Cranial nerve injury? A combination of the above?
It would therefore seem reasonable to propose a prospective randomized-controlled trial of treatment in carotid disease, which is powered to evaluate significant outcomes between three modalities (BMT, CEA plus BMT and CAS plus BMT) for—ideally—symptomatic and asymptomatic disease with pre-agreed outcomes (any stroke and death). So why do we still not have definitive answers despite the multiplicity of trials addressing precisely these issues?
The aim of this chapter is not to exhaustively review the details of every trial, systematic review and pooled meta-analysis; rather it is to identify the difficulties in answering the above questions based on the major trials subsequent to the Cochrane Collaborative Review of 2005. The major outcome of interest throughout all the presented studies is death and stroke of all types.
The Cochrane Review 2005 [1]
This was an attempt to definitively answer—through systematic review and meta-analysis—the questions of who and how to treat in terms of open and endovascular therapy. Five trials including 1,269 patients were reviewed (being a mix of symptomatic and asymptomatic in presentation). Outcomes were reviewed at 30 days and 1 year with the major end-points being any stroke or death and myocardial infarction (30 days) and any stroke or death/cranial nerve injury (1 year). At both 30 days and 1 year there were no significant differences in the Odds Ratios for either any stroke or death (Odds Ratio for stenting 1.33 range 0.86–2.04). There was (as might be expected from the procedural details) a significantly lower Odds Ratio for cranial nerve injury during carotid stenting compared with open surgery (OR 0.13 range 0.06–0.25). We therefore start from the position of neither treatment being superior in terms of the primary end-points, with an advantage in terms of reduced cranial nerve injury. Due to the small numbers and resultant wide confidence intervals, superiority could not be established for either therapy and the recommendation was made to continue with stenting only in the context of controlled trials.
Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Stenosis (EVA-3S) 2008 [2]
Published in 2008, EVA-3S randomized 527 patients with symptomatic disease of 50 % or greater (using the North American Carotid Surgery Trial criteria and defined as an event within 120 days) to either CEA or CAS. Results have been published up to 4 years of follow-up based on an early end to the trial due to safety concerns.
At 30 days the risk of any stroke or death was 3.9 % in the CEA group and 9.6 % in the CAS group, giving a relative risk of the primary end-point in the CAS group of 2.5; an absolute risk increase of 5.7 %. At 6 months the risk of the same end-point was 6.1 % in the CEA group and 11.7 % in the CAS group. Overall the risk of cranial nerve injury was 1.1 % with CAS and 7.7 % with CEA. At 4 years the risk of the primary end-point was 6.2 % for CEA and 11.1 % for CAS. It may therefore be concluded at this stage that the greatest risk of the primary end-point (any stroke or death) occurs in the early perioperative period, and that there is a clear benefit to CEA over CAS in this population. It is also clear, however, that there are multiple outcomes, which may be presented either alone or in combination:
Nonfatal stroke
Death (including fatal stroke)
Full access? Get Clinical Tree








